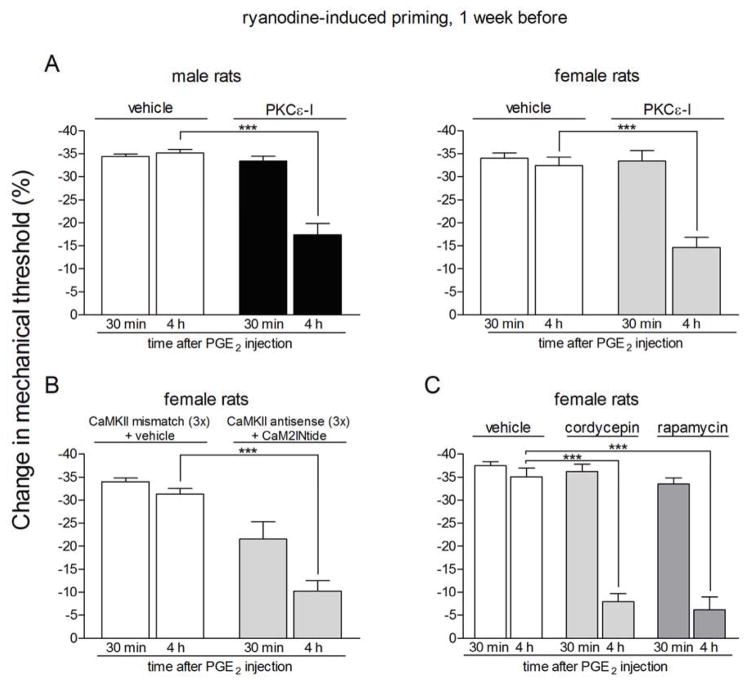
Figure 4. Prolonged phase of PGE2-induced hyperalgesia in rats previously treated with ryanodine is dependent on PKCε, αCaMKII and local protein translation.
Rats primed with intradermal injection of ryanodine (1 μg) on the dorsum of the hind paw one week prior received intradermal injection of PGE2 on the dorsum of the hind paw in the presence or absence of inhibitors of the seconds messengers PKCε (PKCε-I, panel A) or αCaMKII (ODN antisense or mismatch for αCaMKII and the αCaMKII inhibitor CaM2INtide, panel B), and the protein translation inhibitors cordycepin or rapamycin (panel C). Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. PGE2 was injected 5 min (panel A) or 15 min (panels B and C) after the inhibitors. We observed, in all cases, significant attenuation of the hyperalgesia induced by PGE2 at the 4th h (***p < 0.001) in the groups treated with the inhibitors when compared to the vehicle groups (panel A, male rats: F1,10 = 36.03, p = 0.0001; female rats: F1,10 = 18.76, p < 0.0015; panel B, F1,10 = 56.01, p < 0.0001; panel C, F2,15 = 39.30, p < 0.0001, two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), indicating a role of PKCε, αCaMKII and local protein translation in the prolongation of PGE2 hyperalgesia in the ryanodine-induced hyperalgesic priming.