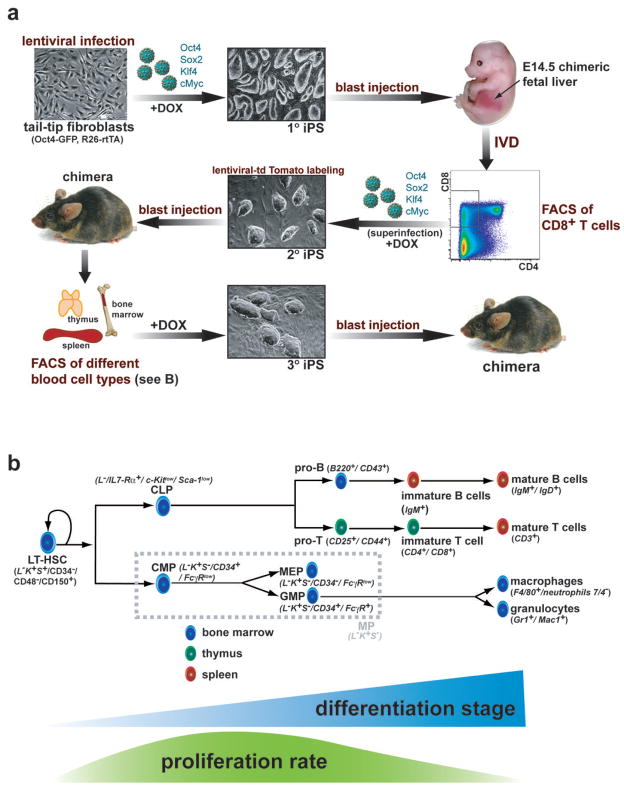
Figure 1. Development of a transgenic system for inducible expression of Oct4, Sox2, Klf4 and cMyc in the murine hematopoietic system.
(a) Strategy used to reprogram different cell types of the hematopoetic system. Tail tip fibroblasts heterozygous for the ROSA26-M2rtTA and Oct4-GFP knock-in alleles were infected with four different doxcycline (dox) inducible lentiviruses encoding Oct4, Sox2, Klf4 and cMyc. Resulting primary (1°) iPS cells were injected into murine blastocytes and fetal liver was isolated from E14.5 embryos, in vitro differentiated (IVD) by stromal co-culture and sorted for emerging CD8 positive (+) T cells by fluorescent-activated cell sorting (FACS). CD8+ T cells were optionally superinfected with the four dox-inducible lentiviruses to ensure strongest possible transgene expression and subsequently cultured on dox for 12 days. Resulting secondary (2°) iPS cells were labeled with a lentivirus constitutively expressing tdTomato and injected into blastocytes to generate adult chimeras. tdTomato+ hematopoietic cells at different stages of differentiation (see Figure 1B) were isolated by FACS from spleen, thymus and bone marrow (BM), induced with dox and resulting tertiary (3°) iPS cell lines were used for molecular and functional analyses including rearrangement and pluripotency assays. (b) Scheme of hematopoiesis with emphasis on the cell populations used in this study. Blue triangle indicates progressive differentiation from LT-HSCs over progenitors to terminally differentiated cells. Green curve illustrates proliferation rate, which is highest in progenitors and lowest in quiescent LT-HSCs and most terminally differentiated cells. LT-HSCs, long-term hematopoietic stem cells; MPs, myeloid progenitors (consisting of CMP, common myeloid progenitors, MEP, megakaryocyte/erythrocyte progenitors and GMPs, granulocyte/macrophage progenitors); CLP, common lymphoid progenitors. Surface markers used for FACS purification are displayed in italic script in brackets, L−K+S+, lin c-Kit+Sca-1+.