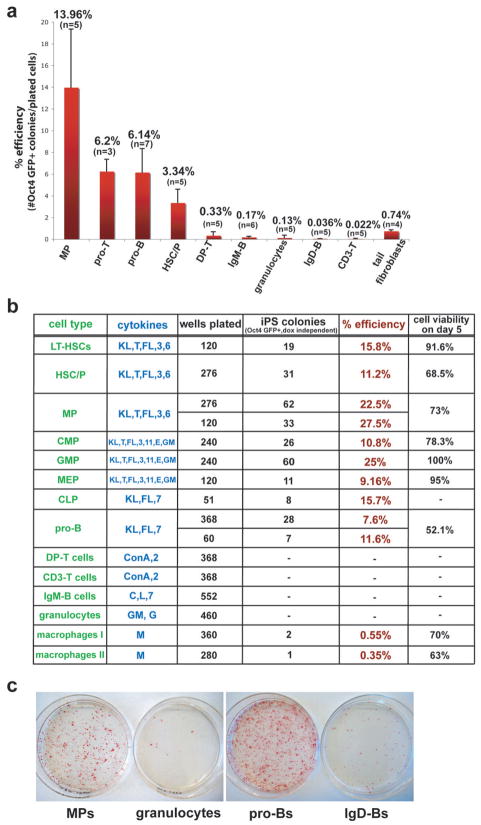
Figure 4. Reprogramming potentials of different hematopoietic cell types into iPS cells.
(a) Average reprogramming efficiencies into iPS cells of different hematopoietic cell types explanted on 10cm dishes on feeder cells in the presence of ES medium supplemented with dox and cytokines. Efficiencies were determined by dividing the number of Oct4-GFP+ colonies that grew in the absence of doxycycline by the number of seeded cells (for details see material and method section). Numbers above each bar represent the mean value for that sample. Bars represent mean ± standard deviation (SD). “n” denotes the number of independent experiments for that particular cell type. Note that the progenitors from different hematopoietic lineages show higher reprogramming potentials compared with any differentiated cell type shown. See figure 1B and legend for details of cell types. (b) Reprogramming efficiencies of different cell types after single cell sorting into 96-well plates. Reprogramming efficiencies were determined by counting Oct4 GFP+ colonies at day 18, 3 days after doxycycline withdrawal. Cell viabilities of individual populations were determined by scoring uninduced cells sorted in 96-well or terasaki plates. “Macrophage I” denotes macrophages directly sorted from BM into 96-well plates while “Macrophage II” denotes macrophages arising from total BM cultures for 5 days in ES medium with M-CSF. KL, Kit-ligand; T, TPO; FL, Flt3-ligand; E, EPO; GM, GM-CSF; G, G-CSF; M, M-CSF; C, CpG; L, LPS; 3, IL-3; 6, IL-6; 7, IL-7; 11, IL-11. (c) AP staining of iPS-like colonies obtained from selected progenitors and mature cell types. Bone marrow MPs (1.5×10^3 cells per 10cm dish), proBs (2×10^3 cells per 10cm dish), granulocytes (1.5×10^3 cells per 10cm dish) and spleen IgD+ mature B cells (1×10^6 cells per 10cm dish) were plated on feeder cells in ES cell media supplemented with dox and cytokines. After 12 days, dox was removed and plates were stained for AP activity at day 14.