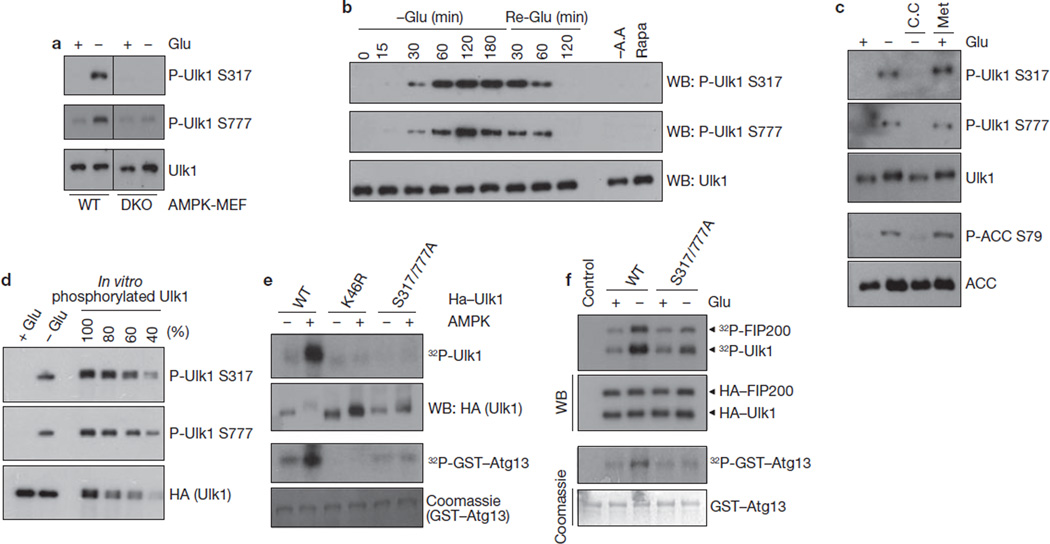
Figure 3.
AMPK-dependent Ulk1 Ser 317 and Ser 777 phosphorylation is required for Ulk1 activation in response to glucose starvation. (a) AMPK wild-type or DKO MEFs were starved of glucose (4 h) as indicated. Total cell lysates were probed for Ulk1 protein and phosphorylation. (b) Time course of Ulk1 Ser 317 and Ser 777 phosphorylation in response to glucose starvation/re-addition. MEFs were starved of glucose (−Glu) for the indicated times. After 3 h starvation, the culture was switched to glucose-containing (25 mM) medium and samples were harvested (Re-Glu). In parallel, cells were treated with amino-acid-free (−A.A) medium or 50 nM rapamycin (Rapa) for 3 h. (c) Phosphorylation of Ulk1 Ser 317 and Ser 777 correlates with AMPK activity. MEFs were starved of glucose (4 h) as indicated in the presence or absence of 20 µM compound C (C.C). In parallel, cells were treated with 2 mM Metformin (Met, 2 h) in glucose-rich medium. Phosphorylation of ACC S79 was tested as a positive control for AMPK activation. (d) Ulk1 is highly phosphorylated at Ser 317 and Ser 777 by glucose starvation in vivo. To determine the Ulk1 phosphorylation level in vivo, immunopurified HA–Ulk1 protein was phosphorylated by AMPK in vitro (100% represents full phosphorylation of Ulk1 by AMPK). In vitro phosphorylated HA–Ulk1 was diluted as indicated, and was immunoblotted along with the immunoprecipitated HA–Ulk1 from cells grown in either glucose-rich (+ Glu) or glucose-free (− Glu, 4 h) medium. The density of the bands was then quantified. By this measurement, approximately 50% of Ulk1 isolated from glucose-starved cells was phosphorylated on Ser 317 and Ser 777. (e) The indicated HA–Ulk1 proteins were immunopurified from transfected HEK293 cells grown in high-glucose medium, and then incubated with AMPK in the presence of cold ATP for 15 min in vitro. After the reaction, AMPK was removed by extensive washing, the resulting Ulk1 immuno-complexes were assayed for kinase activity in the presence of 32P-ATP. (f) HA–Ulk1 proteins (wild type or S317/777A mutant) were immunoprecipitated from the transfected HEK293 cells, which were incubated with or without glucose (4 h) before lysis. An in vitro kinase reaction was performed in the presence of GST–ATG13 and FIP200. Uncropped images of blots are shown in Supplementary Fig. S5.