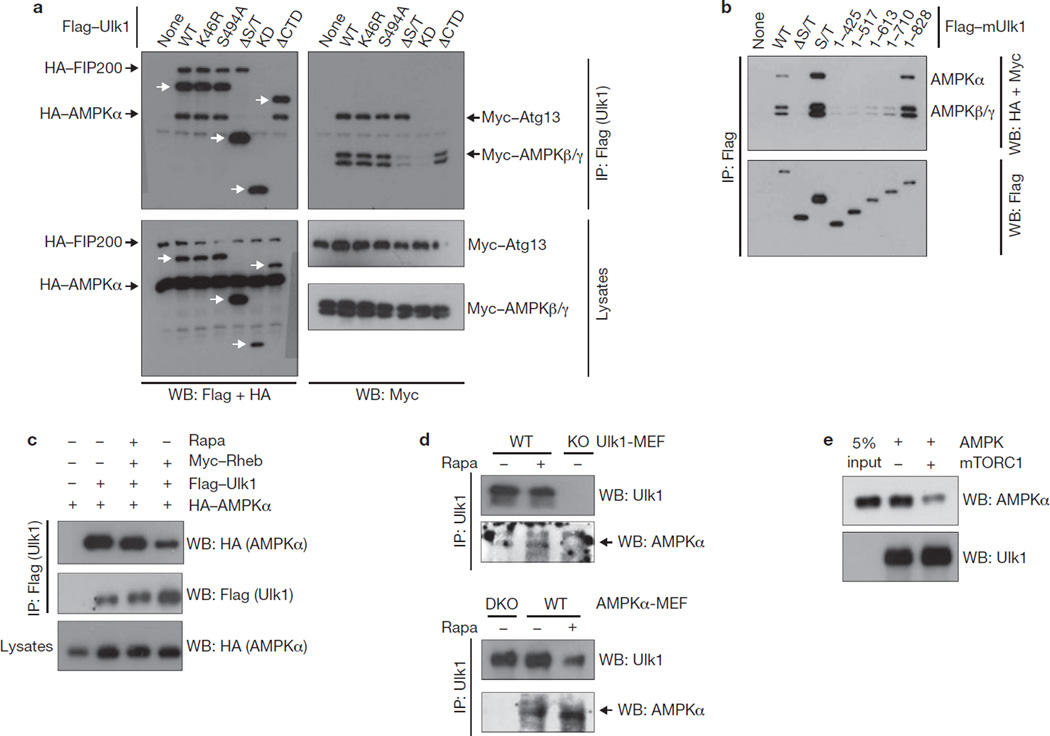
Figure 4.
mTORC1 disrupts the Ulk1–AMPK interaction. (a) AMPK interacts with Ulk1. HEK293 cells were transfected with the various Flag–Ulk1 deletion mutants together with AMPK α/β/γ, Atg13 and FIP200. Flag–Ulk1 protein (indicated by white arrows) was immunoprecipitated and co-immunoprecipitation of AMPK α/β/γ, Atg13 and FIP200 were examined by western blots. (b) Deletion analysis of Ulk1 regions responsible for AMPK interaction. The indicated Flag–Ulk1 truncation mutants were immunoprecipitated from transfected HEK293 cells co-expressing AMPK complex (α/β/γ). Co-immunoprecipitation of AMPK subunits was determined by western blots. (c) Rheb inhibits the Ulk1–AMPK interaction. HA–AMPKα, Flag–Ulk1 and Myc–Rheb were co-transfected into HEK293 cells as indicated. Cells were treated with or without rapamycin (50 nM Rapa) for 1 h before lysis. Flag–Ulk1 was immunoprecipitated and co-immunoprecipitates of AMPKα were determined by western blot. (d) Rapamycin treatment enhances the interaction of endogenous Ulk1 and AMPK. Endogenous Ulk1 proteins were immunoprecipitated from either Ulk1 or AMPK wild-type and knockout (single-knockout; KO or double-knockout; DKO) MEFs. Treatment with 50 nM rapamycin for 1 h is indicated (Rapa). Co-immunoprecipitation of endogenous AMPKα protein was determined by western blot. The arrow indicates AMPKα protein. (e) Phosphorylation by mTORC1 inhibits the ability of Ulk1 to bind AMPK in vitro. CBP/SBP–Ulk1 was purified from transfected HEK293 cells by streptavidin beads and the Ulk1–bead complex was incubated with mTORC1, which was prepared by Raptor immunoprecipitation, in the presence of cold ATP, as indicated. The resulting Ulk1 complex was incubated with the cell lysates containing AMPK, then extensively washed. The Ulk1 and associated AMPKα were detected by western blot. Uncropped images of blots are shown in Supplementary Fig. S5.