Abstract
The transcription factor cMyb has well known functions in vertebrate hematopoiesis, but little was known about its distribution or function at early developmental stages. Here, we show that cMyb transcripts are present at the neural plate during gastrulation in chick embryos. cMyb expression then resolves to the cranial neural folds and is maintained in early migrating cranial neural crest cells during and after neurulation. Morpholino-mediated knock-down of cMyb reduces expression of Pax7 and Twist at the neural plate border, as well as reducing expression of neural crest specifier genes Snail2 and Sox10 and completely eliminating expression of Ets1. On the other hand, its loss results in abnormal maintenance of Zic1, but little or no effect on other neural crest specifier genes like FoxD3 or Sox9. These results place cMyb in a critical hierarchical position within the cranial neural crest cell gene regulatory network, likely directly inhibiting Zic1 and upstream of Ets1 and some, but not all, neural crest specifier genes.
1. Introduction
cMyb is an important transcriptional regulator with diverse functions in the hematopoeitic system. It maintains T cells and other progenitors in a proliferating and immature state (Allen et al. 1999). Interestingly, neural crest cells in the developing embryo share many transcription factors with other stem cell populations, particularly those used in the hematopoietic and immune systems.
Formation of the neural crest begins at the neural plate border during gastrulation stages (Basch et al., 2006), and appears to involve a sequential series of gene regulatory interactions (Betancur, 2010b; Milet and Monsoro-Burq, 2012). In the cranial region, these events are initiated by inductive signals like BMPs and Wnts that set up a domain at the neural plate border that expresses marker genes including Msx, Pax3/7, Twist and Zic genes. These cumulatively render the border region distinct from the neural plate and non-neural ectoderm. Some time later, cranial neural crest cells first become identifiable as a discrete population of premigratory cells within the dorsal neural tube, by their expression of a combination of neural crest specifier genes including Slug/Snail2, FoxD3, and SoxE genes. However, little is known about events that occur between establishment of the neural plate border and the appearance of bona fide neural crest cells. Direct connections between neural plate border genes like Pax7 and neural crest specifier genes like FoxD3 are only now becoming elucidated (Simoes-Costa et al., 2012). In this context, it is interesting to note that cMyb has been shown to be a direct input into the neural crest specifier gene Sox10 (Betancur et al., 2010a), though it had not been previously known to be present or functional in the premigratory cranial neural crest.
Over-expression of cMyb in the trunk neural tube upregulates Msx1 and Snail2, interpreted as evidence that cMyb may participate in BMP4 signaling input into the epithelial to mesenchymal transition of trunk neural crest (Karafiat et al. 2005). Interestingly, cMyb has been shown to have later effects on differentiation of neural crest derivatives. For example, it appears to influence melanocyte fate by regulating c-kit (Karafiat et al. 2007).
To better define cMyb’s role in the context of neural crest gene regulation, focusing on the cranial neural crest, we have performed a detailed analysis of its expression and function from gastrulation throughout neurulation. The results show that loss of cMyb reduces expression of neural plate border genes Pax7 and Twist, increases Zic1, and reduces or eliminates neural crest specifier genes, Ets1, Sox10 and Snail2, but not FoxD3 or Sox9, expression levels. These findings help identify the position of cMyb within the hierarchy of the cranial neural crest gene regulatory network.
2 Results
2.1 Isolation of full-length chick cMyb
As a first step in understanding the possible early functions of this gene in embryonic patterning, we isolated the chick homologue of cMyb and examined its expression from gastrulation through early stages of nervous system development in the chick. The full-length chick homologue of cMyb was obtained by a degenerate RT-PCR approach. Comparative analysis of the aligned sequences reveals that chick cMyb shares 80% identity with the mouse protein, 82% with human, and 75% identity with the Xenopus homolog at the amino acid level.
2.2 Expression pattern of cMyb in the early chick embryo during neural crest formation
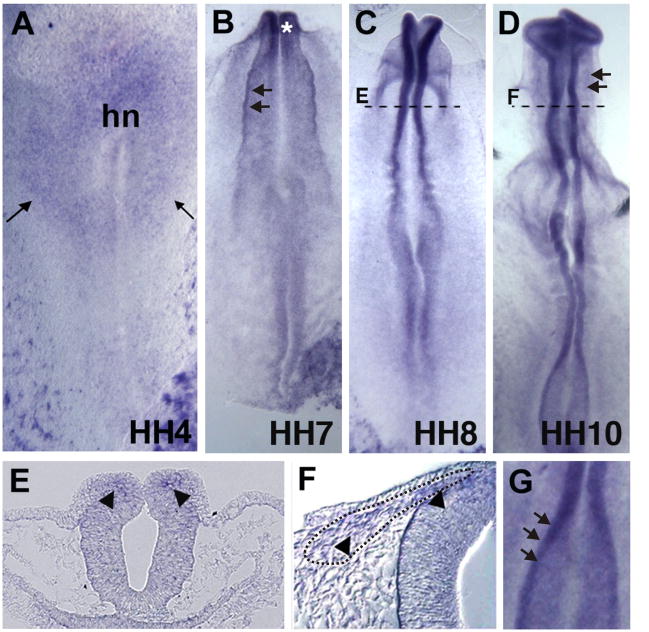
The spatiotemporal expression pattern of chick cMyb was studied by whole mount in situ hybridization beginning at gastrulation to early organogenesis stages. From gastrulation through early neurulation (stages 4–6), we find that cMyb is expressed at the neural plate border and neural plate, as well as in presumptive blood islands (Figure 1A). As neurulation begins, it is expressed in the elevating neural folds (arrows on Fig. 1B), as well as more faintly in the neuroepithelium. Expression is maintained in the presumptive neural crest forming region, accumulating in the neural folds by HH7-8, with strongest expression at the dorsal margins containing neural crest precursors (Figs.1C, E). At HH10, transcripts are seen in neural crest cells delaminating and emigrating from the cranial neural tube (Fig. 1D, F). In the trunk, cMyb expression is detected in the elevating neural folds (arrows in Fig. 1G) as well as the neural plate. Thus, cMyb is expressed early at the neural plate border and in presumptive neural crest cells.
Figure 1. cMyb pattern of expression in the presumptive neural crest territory.
A) cMyb is detected in the neural plate and neural plate border (arrows) at HH4 as well as in blood islands. B) At HH7, cMyb is expressed within the raising neural plate folds, which are beginning to close in the anterior end of the embryo (asterisk). C) By HH8, cMyb is confined to dorsal neural folds containing precursors to cranial neural crest cells by HH8. D) At HH10, cMyb is observed in migrating neural crest. E) Section at dotted line of C showing expression of cMyb in the neural folds (arrowheads). F) Section through dotted line of D showing cMyb expression in migrating neural crest cells (arrowheads). G) Expression of cMyb in the trunk neural folds of a HH10 chicken embryo. Hn = Hensen’s node.
2.3 Effects of cMyb knock-down on neural plate border and neural crest specifier expression
cMyb protein production was perturbed by unilaterally electroporating FITC-tagged antisense morpholino oligonucleotide into one side of HH4 embryo and assaying the subsequent effects on expression of neural crest markers. In particular, we focused on those whose expression precedes that of Sox10. While there was no strong effect on expression of Sox9 (Fig. 2A; n=10/12) or FoxD3 (Fig. 2B; n=8/11), the results show that knock-down of cMyb decreases Snail2 expression (Fig. 2C n=9/13). Even more striking, cMyb loss completely abolishes Ets1 expression (Fig. 2D; n=11/12). To demonstrate specificity of the effect, we co-electroporated cMyb-morpholino with a cMyb mRNA expression construct; the results show that this largely rescues the loss of Ets1 expression caused by cMyb morpholino alone (100% of embryos rescued, n=3/3) (Fig. 2E).
Figure 2. Effects of cMyb mediated morpholino knock-down on neural crest specifier gene expression analyzed at embryonic stage HH8 to HH8+.
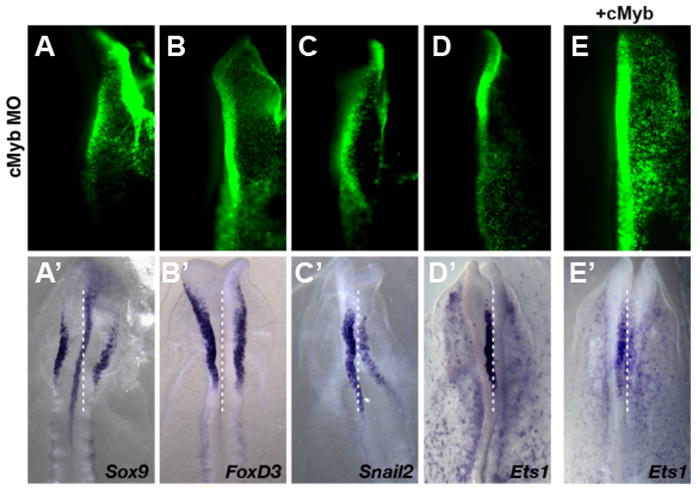
FITC-labeled morpholino to cMyb (A–E) was introduced onto the right side of each embryo, with the contralateral side serving as an internal control. Loss of cMyb does not affect the expression of Sox9 (A′) or FoxD3 (B′) but causes reduction of Snail2/Slug (C′) and completely abolishes Ets1 (D′) expression. Co-electroporation of cMyb morpholino plus mRNA encoding cMyb partially rescued to loss of Ets1 expression (E′) on the right injected side.
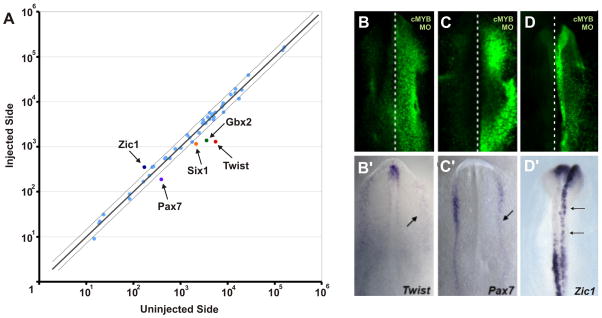
Since cMyb is expressed very early at the neural plate border, we investigated whether perturbing cMyb at stage 4 using antisense morpholino alters expression of other genes expressed at the neural plate border. To test this at a multiplex level, we employed NanoString technology which allows us to quantify the effects of cMyb knock-down of numerous genes simultaneously, using a probe set comprised of 45 neural plate, neural plate border, non-neural ectoderm, and ectodermal placode genes. We directly compared the levels of gene expression on the morpholino injected to the contralateral side of the same embryo. The results show that cMyb knock-down increases the expression of Zic1 while decreasing the expression of Gbx2, Six1, Twist and Pax7 neural plate and neural plate border specifier genes (Fig. 3A). No changes were observed on the expression of GapDH and SDHA among other housekeeping genes and negative control genes. Furthermore, to verify the results and obtain spatial information regarding neural plate border genes, Twist, Pax7 and Zic1, we performed in situ hybridization. The results supported the data obtained with the NanoString analysis and show that both Pax7 and Twist expression are reduced on the morpholino-injected side of the embryo (Fig. 3B, B′, C and C). Interestingly, cMyb knockdown resulted in an increase of Zic1 expression in the dorsal neural folds, which is more prominent in the stage HH9 (Fig. D and D′).
Figure 3. cMyb knock-down decreases expression of neural plate border genes.
A) Nanostring multiplex analysis shows that loss of cMyb causes downregulation of neural plate border genes Pax7 and Twist1, as well as placode markers. In contrast, Zic1 is upregulated. In situ hybridization confirms that the right morpholino-treated side (B, C) has less Pax7 (B′) and Twist1 (C′) expression (arrow) than the control side of the same embryo. Expression of Zic1 is increased in the cranial neural folds of embryos electroporated with cMyb morpholino (D′).
3 Discussion
By examining the expression pattern of cMyb in the early avian embryo, we find that it is expressed at the neural plate border region. Previously, we identified cMyb as a direct input into the initiation of Sox10 expression at stage 8+ (Betancur 2010a). The present results show that its expression commences much earlier than Sox10. cMyb transcripts are observed during gastrulation, concomitant with early neural crest specifiers such as Pax7, AP-2 or c-Myc. It remains expressed in premigratory neural crest cells within the dorsal head neural folds, as well as in migrating cranial neural crest cells. This raised the intriguing possibility that cMyb may regulate additional genes in the neural crest gene regulatory network. Here, we demonstrate that cMyb differentially regulates expression of several genes, whose onset of expression precedes that of Sox10. cMyb knock-down reduces the expression of Snail and eliminates expression of Ets1, but does not effect other neural crest specifier genes, like Sox9 and FoxD3. The complete loss of Ets1 expression indicates that cMyb is necessary for the expression of Ets1. However, it does not appear to be a primary input into a recently reported cranial crest enhancer for Ets1 (Barembaum and Bronner, 2013), raising the possibility that its regulation of Ets1 is indirect or functions through a different enhancer. Our previous data show that cMyb and Ets1 cooperate to activate Sox10 expression in cranial neural crest cells. The present data suggest that cMyb helps activate Ets1 and they then both help to initiate and maintain expression of Sox10. Later in development, cMyb functions to specify melanocyte fate by regulating c-kit (Karafiat et al., 2007), suggesting that cMyb plays multiple spatially and temporally distinct roles in the neural crest.
Our NanoString data suggest that cMyb is involved in the regulation of neural and neural plate border genes Gbx2, Six1, Twist and Pax7 in the late gastrula embryo. Interestingly, we find that loss of cMyb results in abnormal maintenance of Zic1 in the cranial neural folds, which is normally only expressed at low levels in the head compared with the trunk neural crest. Zic1 is a member of the Zinc-finger containing family of proteins and a well known neural plate border specifier, also expressed in the neural plate border region and in the anterior ectoderm region containing placodal progenitors (Khudyakov et al., 2009). Much of the information about the role of Zic1 in the neural crest gene regulatory network comes from experiments performed in frog and lamprey (Sato et al., 2012; Nikitina et al). The finding that loss of Myb results in upregulation of Zic1 in the cranial neural folds raises the intriguing possibility that cMyb may normally function to repress Zic1 expression in the head. Recently, it has been shown that Zic1 plays an important role in regulating trunk neural crest expression of FoxD3 (Simoes-Costa et al., 2012). Thus the differential effects of cMyb on Ets1 and Zic1 may partially explain the reciprocal expression pattern of Ets1 in the cranial neural folds and Zic1 in the trunk neural folds (Simoes-Costa et al., 2012). We speculate that different levels of Zic1 and Ets1 expression may contribute to differences in developmental potential between cranial and trunk neural crest cells.
In contrast to Zic1, loss of cMyb causes a decrease in expression of Gbx2, and Six1. Gbx2, is a homeobox gene, important for the anterior-posterior patterning of the neural axis, previously reported to be expressed in the ectodermal regions that includes the future neural plate border in Xenopus embryos (Li et al., 2009). Six1, on the other hand, is expressed in the anterior neural plate, marking a region containing mostly placodal precursors (Streit, 2004). More recently, Sato and colleagues (2012) detected seven functional Six1 enhancers and through mutational analysis, identified inputs from Sox, Pax, Fox, Wnt/Lef1 and an autoregulatory input from Six1 that potentially integrate through these enhancers to regulate Six1 expression at different times of placode development (Sato et al., 2012). Nevertheless, their study did not identify a functional binding motif for cMyb, suggesting that perhaps the reduction on Six1 expression observed in our NanoString data might be indirect and possibility mediated by other transcription factors, such Pax7.
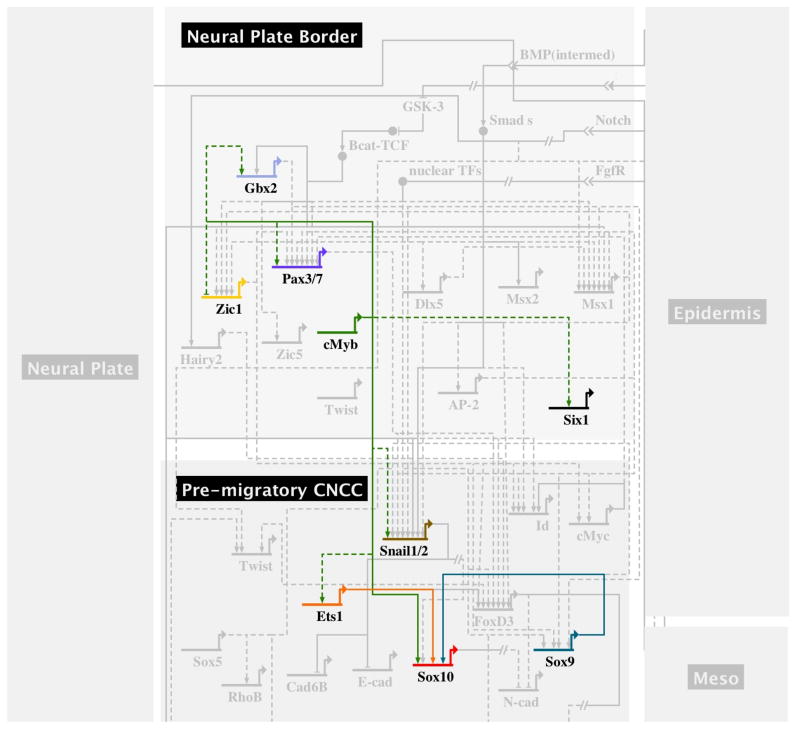
In summary, our data help to elaborate the gene regulatory underlying neural crest formation (Figure 4). Most notably, we show that cMyb functions early in neural crest development by partially regulating Pax7 and Twist at the neural plate border. These factors are part of a subgroup of transcription factors that include AP-2a, Snail1/2, Id, c-Myc, among others, and that are expressed well before neural crest progenitors become apparent. Most notably, our data suggest that cMyb plays a critical role in regulating cranial neural crest expression of the neural crest specifier gene, Ets1, which together with cMyb itself, is a direct activator of initial Sox10 expression in the premigratory and emigrating cranial neural crest cells.
Figure 4.
Schematic representation of the neural crest gene regulatory network illustrating the position of cMyb therein.
This new information allows us to place cMyb within the gene regulatory network hierarchy underlying neural crest cell formation. Furthermore, combining morpholino knockdown and multiplex NanoString plus in situ hybridization assays guides in the establishment of linkages between the genes within the neural crest cell gene regulatory network (Fig. 4), thus helping to unravel the complex interacting events for testing by in depth cis-regulatory analyses.
4 Experimental Procedures
4.1 In situ hybridization
Sox9 and Sox10 probes were prepared using full length cDNA constructs (a gift from Yi-Chuan Cheng) as a template. Other digoxigenin-labeled antisense RNA probes were prepared from chicken EST clones obtained from (ARK Genomics and MRC geneservice). Sox10 template was digested with HindIII, while all EST clones were linearized using NotI restriction enzyme. All antisense RNA probes were synthesized using T3 RNA polymerase, according to standard protocols. Whole-mount in situ hybridizations were performed as previously described (Wilkinson, 1992). Fluorescent in situ procedure using GFP probe was adapted from Acloque et al. (2008).
4.2 Embryos culture and electroporation
Fertilized chicken eggs were obtained from AA Laboratories, Westminster, CA and Chino Valley Ranchers, Arcadia, CA and incubated at 37–38°C for approximately 24–40hrs, depending on the desired stage. The embryos were staged according to the criteria of Hamburger and Hamilton (1992).
Chicken embryos were electroporated at stages HH4-8 to target the cranial neural crest cell population. Morpholinos were introduced unilaterally to cover one side of the epiblast of the early chicken embryo (right to the primitive streak). The final molar concentration of each morpholino oligonucleotide ranged from 1–2 mM.
4.3 Morpholinos
To target the translation initiation site, FITC-labelled morpholino antisense oligonucleotides or controls (Gene Tools, Philomath, OR, USA) were designed following general manufacturer’s as follows:
cMyb_5′-ATGGCCGCGAGCTCCGCGTGCAGAT-3′;
Control_5′-ATGGCCTCGGAGCTGGAGAGCCTCA-3′.
4.4 NanoString nCounter
Embryos at primitive streak stage were unilaterally electroporated with antisense morpholino against cMyb on the right side of the embryo and control morpholino on the left side. Embryos were cultured ex vivo and allowed to grow for 24 hours. Next, each individual half of the embryo was processed and the total RNA obtained from each side was prepared as previously described (Strobl-Mazzulla et al., 2010).
4.5 Microscopy and inmunohistochemistry
The electroporated embryos were collected at stages 8–10, fixed in 4% paraformaldehyde overnight and then washed three times in PBS at room temperature. A Zeiss Axioskop2 Plus fluorescence microscope equipped with the AxioVision software was employed to image the embryos. Images were processed using Adobe Photoshop CS2. After imaging, embryos were cryo-protected in two steps: 15%sucrose/PBS and 7.5%gelatin/15% sucrose/PBS, equilibrated and mounted in 20% gelatin/PBS and frozen in liquid nitrogen. 12μm cryosections were collected on Super Frost Plus slides (Fischer Scientific, Pittsburgh, PA) and de-gelatinized for 2x 10 minutes at 42°C in PBS. To intensify EGFP signal, the sections were washed 4x in PBS for 5 minutes, blocked for 1 hour in 10% Donkey serum / PBTW (PBS/0.1% Tween-20) and stained with 1:1000 anti-GFP primary antibody (Abcam Inc., Cambridge, MA) followed by 1:2000 Donkey anti-goat Alexa-Fluor 488-conjugated secondary antibody (Molecular probes). Sections were subsequently washed, cover slipped and imaged using the same imaging procedure described for the whole-mounts.
Highlights.
cMyb is expressed at the neural plate border and presumptive neural crest region
cMyb knock-down results in decrease of neural crest specifier genes Ets1, Sox10 and Snail2
Repression of Zic1 in the cranial neural folds is mediated by cMyb
Acknowledgments
This work is supported by USPHS P01 HD037105
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acloque H, Wilkinson DG, Nieto MA. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods Cell Biol. 2008;87:169–185. doi: 10.1016/S0091-679X(08)00209-4. [DOI] [PubMed] [Google Scholar]
- Allen RD, 3rd, Bender TP, Siu G. c-Myb is essential for early T cell development. Genes Dev. 1999;13:1073–1078. doi: 10.1101/gad.13.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441:218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- Barembaum M, Bronner ME. Identification and dissection of a key enhancer mediating cranial neural crest specific expression of transcription factor, Ets-1. Dev Biol. 2013 doi: 10.1016/j.ydbio.2013.08.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur PA, Bronner-Fraser M, Sauka-Spengler T. A key regulatory enhancer for cranial neural crest: Genomic code for Sox10. Proc Natl Acad Sci U S A. 2010a;107:3570–5. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol. 2010b;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karafiat V, Dvorakova M, Pajer P, Cermak V, Dvorak M. Melanocyte fate in neural crest is triggered by Myb proteins through activation of c-kit. Cell Mol Life Sci. 2007;64:2975–2984. doi: 10.1007/s00018-007-7330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karafiat V, Dvorakova M, Krejci E, Kralova J, Pajer P, Snajdr P, Mandikova S, Bartunek P, Grim M, Dvorak M. Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cell Mol Life Sci. 2005;62:2516–2525. doi: 10.1007/s00018-005-5297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136:3267–78. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milet C, Monsoro-Burq AH. Neural crest induction at the neural plate border in vertebrates. Dev Biol. 2012;366:22–33. doi: 10.1016/j.ydbio.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene regulatory network. Proc Natl Acad Sci USA. 2008;105:20083–8. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Ikeda K, Shioi G, Nakao K, Yajima H, Kawakami K. Regulation of Six1 expression by evolutionarily conserved enhancers in tetrapods. Dev Biol. 2012;368:95–108. doi: 10.1016/j.ydbio.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Strobl-Mazzulla, Sauka-Spengler T, Bronner-Fraser M. Histone demethylase JmjD2A regulates neural crest specification. Dev Cell. 2010;19:460–8. doi: 10.1016/j.devcel.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridisation of vertebrate embryos. In: Wilkinson DG, editor. In situ Hybridization: A Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]