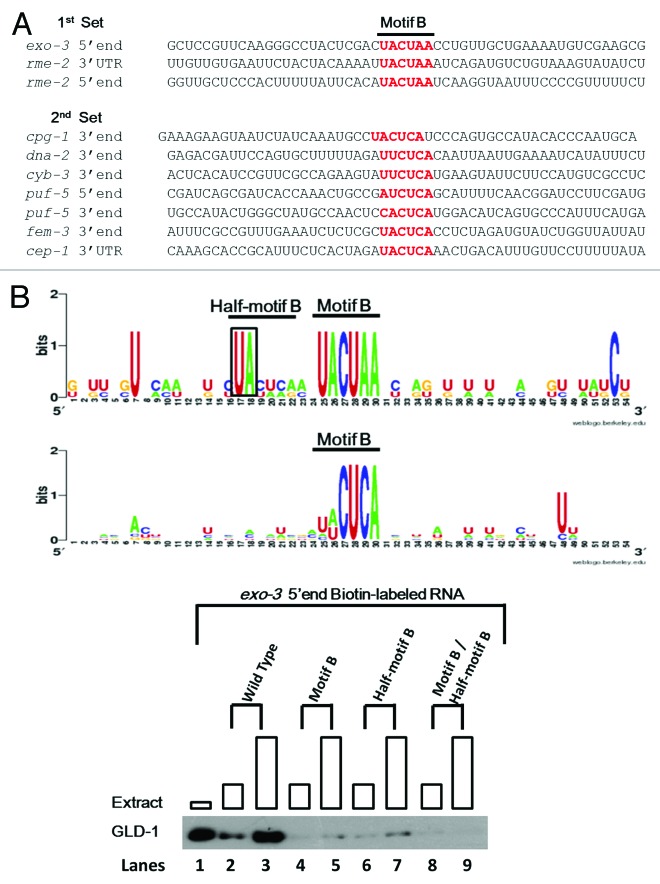
Figure 3. The identification of the Half-motif B and its importance in GLD-1 binding. (A) The possibility that sequences surrounding Motif B may contribute in a context-dependent manner to GLD-1 binding was examined. Two sets of sequences were compiled. The first set contains three GBRs where mutations of the Motif B reduced or eliminated GLD-1 binding, indicating that the Motif Bs in these GBR are important for GLD-1 binding. The second set contains the seven GBRs where GLD-1 still binds well even though the Motif Bs are mutated, indicating that the Motif Bs in these regions have no biochemical importance. Next, these two data sets were aligned so that Motif B was centered within the alignment with additional ~25 nucleotides flanking either side of the Motif Bs. (B) With the hypothesis that adjacent sequences important for GLD-1 recognition should be conserved, two independent sequence alignments were performed to see if any nucleotides were conserved in the first but not in the second set. Sequence alignment matrixes of the two sets are shown. Motif B is highly conserved in both data sets. Also, a Uridine-Adenine (UA) site just upstream of Motif B is highly conserved in the sequence alignment of the first set, which is not detected in the sequence alignment of the second set. Sequences that immediately follow the conserved UA site in the first set represent a weakly conserved Motif B, (UA C/U U/A C/A G/A) and is therefore designated as a Half-motif B. (C) The importance of Half-motif B for GLD-1/RNA interaction was examined. A western blot of the GLD-1/ Biotin-RNA pull-down assay with the wild-type R09B3.1 5′end and corresponding mutations is shown. The first lane shows GLD-1 in cytosolic extract. Lanes 2 through 9 represent binding reactions with wild-type and mutated R09B3.1 5′end biotin-labeled RNAs (400 ng) with increasing amount of cytosolic extract (50 and 150 ug). The conserved “UA” dinucleotide of the Half-motif B was mutated to “AU” and the Motif B (“UACUAA”) was mutated “UAGAUU” within R09B3.1 5′end (see Fig. 3B). The mutation of the Half-motif B reduces GLD-1 binding as significantly as the mutation of the Motif B compared with the wild-type. A double mutation of both motifs appears to reduce GLD-1 binding further, where the residual GLD-1 bands are no longer detected. Boxes are each lane are proportional to the amount of extract used.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.