Abstract
The resistance arteries and arterioles are the vascular components of the circulatory system where the greatest drop in blood pressure takes place. Consequently these vessels play a preponderant role in the regulation of blood flow and the modulation of blood pressure. For this reason, the inward remodeling process of the resistance vasculature, as it occurs in hypertension, has profound consequences on the incidence of life threatening cardiovascular events. In this manuscript, we review some of the most prominent characteristics of inwardly remodeled resistance arteries including their changes in vascular passive diameter, wall thickness and elastic properties. Then we explore the known contribution of the different components of the vascular wall to the characteristics of inwardly remodeled vessels, and pay particular attention to the role the vascular smooth muscle actin cytoskeleton may play on the initial stages of the remodeling process. We end by proposing potential ways by which many of the factors and mechanisms known to participate in the inward remodeling process may be associated with cytoskeletal modifications and participate in reducing the passive diameter of resistance vessels.
Keywords: hypertension, stress, strain, stiffness, elasticity, matrix metalloproteinases, transglutaminase, actin polymerization, Rho, Rac
INTRODUCTION
Cardiovascular diseases are the most important life-threatening health conditions today, with hypertension and cerebrovascular disease being two of the most predominant ones, and projected to rapidly increase in the next few years [50]. The majority of cases of hypertension are considered essential, as no apparent cause can be established for the elevated arterial pressure. Given the importance and the impact that hypertension has in human lives, a detailed study and better understanding of its pathophysiology is warranted, especially on the role that the structure and function of resistance vessels play in it, because recent studies indicate that remodeling of resistance arteries is one of the earliest detectable parameters that predict subsequent life threatening cardiovascular events [52, 67]. In this article, we present a review of some of the main results from several studies that focused on the characterization of the most prominent structural changes that occur in the resistance vasculature in hypertension, i.e., the inward eutrophic remodeling of arterioles [4, 5, 49, 74, 80]. We will present evidence that the inward eutrophic remodeling of arterioles is closely associated with augmented active tone induced via prolonged agonist-induced vasoconstriction, which stimulates structural modifications in the arteriolar wall, leading to changes in the elastic and mechanical properties of the vascular wall. Evidence indicates that during the initial stages of the remodeling process these changes occur mainly at the level of the actin cytoskeleton and are associated with the repositioning of vascular smooth muscle cells, actin polymerization pathways, and the accumulation of fibrillar (F)-actin. As results indicate that blockade of Rho and Rac-1 associated pathways prevent prolonged vasoconstriction from inducing inward eutrophic remodeling, we end with a hypothetical model of how these small GTPases may contribute to the remodeling process.
STRUCTURE AND ELASTIC PROPERTIES OF RESISTANCE ARTERIES
The structure and composition of arterioles, and blood vessels in general, has been widely studied and described [44, 63, 64]. However, much remains to be understood about the mechanics and interactions of the different components in the vessel wall. Resistance arteries play a preponderant role in the regulation of blood flow and modulation of blood pressure in the cardiovascular system. For this reason, any structural change that occurs in the resistance vasculature, e.g., the narrowing of blood vessels, the thickening of vessel walls, increased/decreased stiffness, etc., can impact the mechanics of the arteriolar wall, the control of the cardiovascular system and the development of cardiovascular diseases [20]. To characterize the mechanical properties of the blood vessel wall, the most common parameters studied are the stress, strain, and elastic modulus of vessels placed under passive condition.
Circumferential Stress
The stress profile characterizes the internal forces exerted in between the individual components of a continuous material, for example, the forces in between adjacent smooth muscle cells in the vessel wall, due to an external force (e.g., intraluminal pressure). The stress represents an average force per unit-area. In the study of blood vessels associated with hypertension, the circumferential stress is more often examined as it contains information on the dimensions of the vascular luminal diameter and wall thickness at a given pressure, which are dimensions commonly affected by changes in blood pressure. In the microcirculation, circumferential stress is commonly expressed in dynes/cm2, and can be written as σθ,i = (Pi· ri)/τi = (Pi· Di)/2τi, where σθ,i is the circumferential stress at the ith level of intraluminal pressure with its respective vascular diameter and wall thickness, Pi is the intraluminal pressure, and Di and τi are the internal diameter and the wall thickness at a given pressure, respectively. When studying resistance arteries, it is commonly assumed that the arteriolar wall volume remains constant under changes in pressure, at a fixed vessel length, this would result in a CSA (cross-sectional area) that remains constant. [8-10] In this case, the wall thickness could be calculated and expressed in terms of the CSA and the internal diameter as follows
Strain
Strain is a normalized measure of the displacement between the components of a continuous material. In the case of blood vessels, the strain (circumferential strain) represents a measure of the change in internal diameter due to a change in intraluminal pressure normalized by a reference diameter, which normally is the diameter measurable at the lowest possible pressure (because vessels pressurized at 0 mmHg would collapse, pressures between 5-10 mmHg are commonly used as reference). Being a normalized measure, the strain has no-units, and it can be written as εi = (Di − D0) /D0, where Di is the diameter at a given pressure and D0 is the reference diameter.
Modulus of elasticity
The modulus of elasticity, also known as tangential elastic modulus, is a parameter that measures the stiffness of an elastic continuous material. Mathematically it represents the point-by-point slope in a strain vs. stress curve. At every single point in this curve, the elastic modulus can be calculated as ET,i = σθ,i/εi.
Traditionally the extracellular matrix components of the vascular wall are considered the major contributors to the elastic properties of arterioles under passive conditions [34, 35, 79]. Due to their relative amount and elastic properties, collagen and elastin are the extracellular matrix components with major influence in vascular wall mechanics [79]. However, to the best of our knowledge there are no systematic studies that have experimentally established the proportion by which different cellular and extracellular components of arterioles contribute to the elastic properties of the vascular wall. This is particularly important to establish in arterioles where smooth muscle is the major component of the vascular wall.
Anatomically, the arteriolar wall is traditionally segmented from the lumen out into three different parts: The initma is composed of endothelial cells and a basement membrane. Endothelial cells are major contributors to the control of vascular tone. Evidence indicates that endothelial cells modify their intracellular (i.e., cytoskeletal) structure based on the shear stress they are exposed to as blood flows in the vascular lumen. It has also been shown that a number of mechanical and physiological mechanisms, and intracellular/cell-cell interactions are shear-stress mediated (e.g., production of nitric oxide and other vasodilator compounds, expression of nitric oxide synthase, presence and activity of adhesions between adjacent endothelial cells, cell membrane stability, cytoskeletal remodeling, etc.) [42, 43, 70, 75, 76, 78]. The direct contribution of endothelial cells themselves to the elastic properties of the vascular under passive conditions, however, is likely to be minimal as indicated by experiments in which the vascular intima of arterioles has been denuded [25].
The media, which in arterioles consists mostly of one or two layers of smooth muscle cells, is in charge of controlling the functional vascular diameter via mechanisms of cellular contraction and relaxation. Recent results from our laboratory also suggest that in the early stages of the inward eutrophic remodeling process they provide a significant contribution to the passive diameter of arterioles [74]. An additional component of the media in arterioles is the internal elastic lamina. The elastic laminas are constituted primarily of elastin fibers, which provide blood vessels with recoiling properties that allow them to expand and recover to their original diameter when external forces are applied and withdrawn. In inwardly remodeled arteries it has been shown that the fenestra (holes) present in the internal elastic lamina are reduced in size [13, 14], suggesting that remodeling of elastin may contribute to the reduction in passive diameter observed in inwardly remodeled vessels. In arterioles, the media is the thickest layer in the vascular wall. It contributes in a very important way to wall mechanics under active vasoconstriction. The contractile level of smooth muscle cells, the interactions in between multiple cells, the intracellular structure of the cell (e.g., actin cytoskeleton), and their interactions with the extracellular matrix including elastin molecules in the elastic laminas, will determine one of the major components of the elastic properties of the actively contracted arteriolar wall. The contribution of the media to the circumferential elastic properties of the arteriolar wall under passive conditions, however, appears to be minimal in “normal” arterioles obtained from normotensive rats, as actin cytoskeletal disruption or elastin degradation have no impact on maximal arteriolar passive diameter [21, 74].
The adventitia, the outermost segment of blood vessels, is mainly composed of collagen and fibroblasts, which are embedded within the collagen. This layer is considered to give support and structure to the arteriolar wall. It is considered to be a major contributor to vascular stiffness and elasticity, as collagen disruption severely affects vascular mechanics and is commonly used to dissociate the cellular elements of the wall.
THE INWARD REMODELING OF RESISTANCE ARTERIES
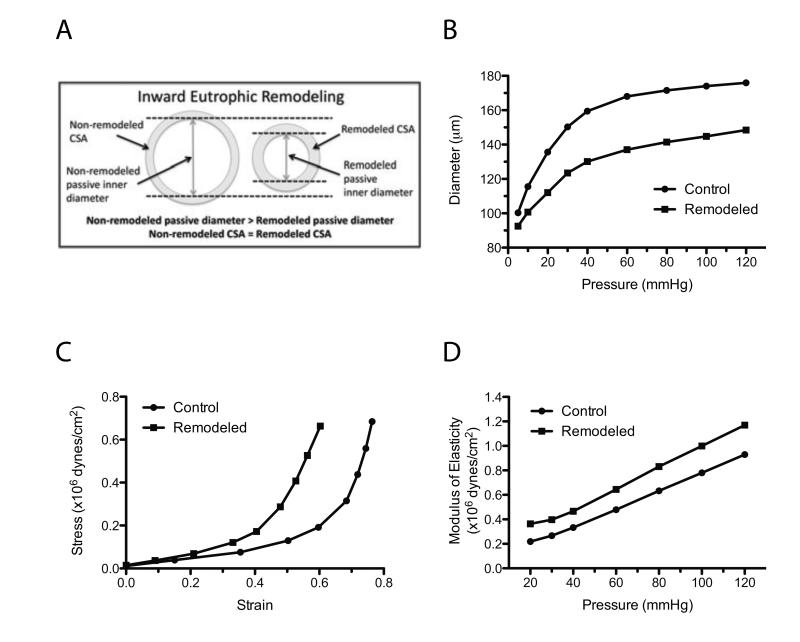
In essential hypertension, inward remodeling is the most commonly observed change in arteriolar structure. It is characterized by a reduced luminal diameter under passive conditions, and further categorized as eutrophic when the cross-sectional area of the vascular wall remains without significant changes (Fig. 1A,B). It has been postulated that inward eutrophic remodeling occurs when resistance vessels exposed to high blood pressure are able to normalize circumferential stress via the repositioning of vascular smooth muscle cells around a smaller luminal diameter, a process that preserves wall cross-sectional area [31, 32, 47]. If this process is insufficient, wall hypertrophy occurs to normalize the circumferential stress of the vascular wall.
Figure 1.
Structural and mechanical characteristics of an inwardly remodeled resistance artery. A) Diagrammatic representation describing the main changes in inner and outer diameters and cross-sectional area observed in the arteriolar wall of arterioles with inward eutrophic remodeling. B) Diagrammatic representation of the intraluminal pressure to passive diameter relationships of a control and an inwardly remodeled arteriole C) Diagrammatic representation of the strain-stress relationships of the control and inwardly remodeled arterioles presented in panel 1B. D) Diagrammatic representation of the moduli of elasticity obtained from the control and inwardly remodeled arterioles presented in panel 1B.
A reduced luminal diameter with a cross-sectional area that remains constant would cause the wall to lumen ratio to be increased, in other words, it would cause materials in the arteriolar wall to rearrange, leading to thickening of the arteriolar wall. An increased media to lumen ratio is an arteriolar feature commonly observed in essential hypertension, and based on the available evidence, is mainly due to rearrangement of the existent normal-sized cells around a smaller luminal diameter [4, 30]. That is, there is no cell hypertrophy or hyperplasia. It is important to consider that any structural modifications in the arteriolar wall would induce changes in the viscoelastic properties and mechanics of the vessel. However, the overall change in vascular mechanics would depend on the viscoelastic characteristics of the materials being modified and the relative amount of these materials in the arteriolar wall.
As an example, let us consider the curves shown in Figure 1, where the elastic characteristics of a control (non-remodeled) and an inwardly eutrophic remodeled arteriole are compared. From the equations for stress, strain and elastic modulus presented in the previous section, we know that the stress is directly proportional to the inner diameter and inversely proportional to wall thickness. In Figure 1B, the inner passive diameter in the remodeled vessels is smaller than the control at all intravascular pressures. The resulting strain-stress relationship of these two vessels are represented in Figure 1C. Notice that the point-by-point circumferential stress at a given level of strain is greater in the remodeled arteriole. However the greatest level of stress achieved at the highest intraluminal pressure is slightly reduced after remodeling because the remodeled vessel is less pliable and distended less at the highest pressure. In the remodeled vessel, the strain has been substantially reduced (i.e., is less distensible) due to the stiffening (increased modulus of elasticity) of the arteriolar wall (Figure 1C, D).
The mechanisms that control inward eutrophic remodeling have not been completely elucidated, in particular those associated with the initial stages of the process. Overall, substantial evidence indicates prolonged vasoconstriction is a primary condition that induces inward remodeling [5, 32, 45, 49]. Whether all stimuli capable of inducing prolonged vasoconstriction can also cause inward eutrophic remodeling has not been clearly established. However, cumulative in vivo and ex vivo studies suggest that prolonged agonist-dependent stimulation for vasoconstriction induces inward remodeling in resistance vessels. Accordingly, prolonged exposure of isolated arterioles to vasoconstrictors such as endothelin-1, norepinephrine, and angiotensin II has been shown to cause inward remodeling [5, 45]. In vivo, prolonged infusion or expression of vasoconstrictor agonists induces inward remodeling as well [19, 32, 85]. A number of results suggest that the level of vasoconstriction achieved by the agonists corresponds to the level of reduction in passive diameter observed in the remodeled vessel [45]. However, this does not appear to be the case for all agonists [45]. Additional studies suggest that, in vivo, the overall influence that vasoconstrictor or vasodilator agonists have on a vascular segment is able to control the remodeling process [51, 69]. This is particularly evident in experiments showing that a diminished vasodilator influence caused by a reduction in blood flow and shear stress-dependent production of nitric oxide and activation of transglutaminase activity causes inward remodeling in resistance arteries [4, 6, 26].
The mechanisms associated with the inward remodeling process achieved either by prolonged exposure to vasoconstrictor agonists or a reduction in blood flow have been associated with processes that involve multiple factors, such as reactive oxygen species (ROS), nitric oxide, Rho, Rac-1, matrix metalloproteinases (MMP), and tissue type transglutaminase (TG2) among others [4, 26, 49, 74]. All of these factors have the potential to affect cytoskeletal and extracellular matrix structures of the vascular wall. However, how these factors participate in the remodeling process both temporally and mechanistically remains to be fully elucidated. Recently we reported that during the early stages of the inward eutrophic remodeling process, nearly 75% of the reduction in passive diameter observed in isolated arterioles constricted for four hours was reversed following actin cytoskeletal disruption. Below we present a brief review of some of the known and proposed roles some of the above mentioned factors play on the actin cytoskeleton and potentially on the inward remodeling process.
VASOCONSTRICTION AND ACTIN POLYMERIZATION
Over the last several years, it has become evident that calcium sensitization and actin polymerization processes participate in the contraction of smooth muscle. These pathways appear to intermingle and collaborate with the classical pathway of constriction induced by increments in intracellular calcium concentration and the subsequent increase in myosin light chain phosphorylation and actomyosin cross bridge cycling (Fig. 2). Studies performed in different types of smooth muscle including airway, vascular, and intestinal muscle indicate that exposure to contractile agonists induces the polymerization of actin and that this actin polymerization is required for the development of force [1, 53, 59, 60, 62, 68, 77]. Importantly, results also indicate that inhibition of actin polymerization by agents such as cytochalasin-D or latrunculin-A do not affect the signaling pathways that regulate myosin light chain phosphorylation during smooth muscle contraction [23, 68].
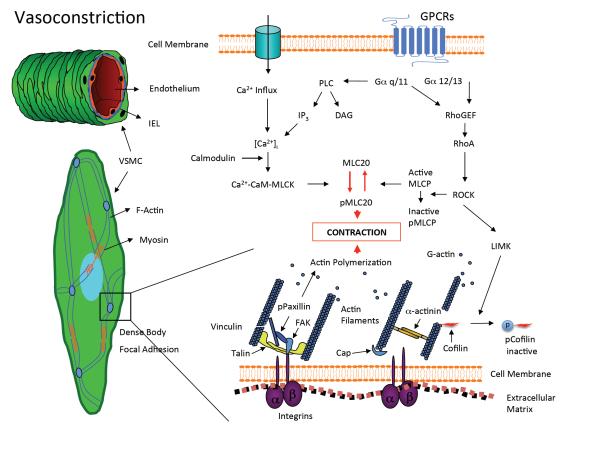
Figure 2.
Vascular smooth muscle intracellular mechanisms for vasoconstriction. Vascular smooth muscle cells (VSMC) located in the medial layer of resistance arteries reduce their length to cause vasoconstriction. This process involves mechanisms associated with the phosphorylation of myosin-light chain (MLC20), and the formation and disruption of actin cytoskeletal structures. The activation of Rho Kinase (ROCK) is an event that potentially links MLC-20 phosphorylation and actin polymerization mechanisms. ROCK inactivates myosin-light chain phosphatase (MLCP) to maintain MLC-20 phosphorylation and constriction. It also deactivates cofilin and its severing action on actin filaments via the activation of LIM kinase (LIMK). Consequently integrin linked actin fibers are able to polymerize and strengthen the cytoskeleton through processes that involve the phosphorylation of paxillin and a number of other focal adhesion proteins with and without kinase activity. GPCRs, G-protein coupled receptors; IEL, internal elastic lamina; PLC, phospholipase C; IP3, inositol triphosphate; DAG, diacyl glycerol; RhoGEF, Rho guanine exchange factor; MLCK, myosin-light chain kinase; FAK, focal adhesion kinase. Figure adapted from references 28, 47.
Evidence suggests that the actin polymerization pathways taking place upon stimulation for constriction serve to strengthen a scaffold of actin fibers (primarily cortical actin) that allows for contractile fibers to exert force appropriately, and shorten the cell [83]. Hypothetically a reversed loosening of these structures occurs during cell relaxation in response to contractile agonists withdrawal or in the presence of relaxing compounds. In support of this hypothesis a recent study reported that the cortical stiffness of isolated vascular smooth muscle cells increases in response to stimulation with the vasoconstrictor agonist angiotensin II, and is reduced upon exposure to a vasodilator [33]. In vascular structures some results suggest that actin polymerization increases as vasoconstriction is prolonged [62]. In accordance with those results, we recently reported that stimulation of isolated arterioles for four hours with the vasoconstrictor agonists norepinephrine and angiotensin II increased the ratio of filamentous (F) to globular monomeric (G) actin obtained by differential centrifugation of the tissue [74]. Our results further suggest that some actin structures formed during the prolonged exposure to the vasoconstrictor agonists are not readily disrupted by withdrawal of the vasoconstrictor agonists, exposure to vasodilator compounds, or removal of calcium. Only the active severing disruption of actin filaments with mycalolide B was able to allow for vascular relaxation to passive diameters similar to those observed before the prolonged exposure to the vasoconstrictor agonists. We hypothesize that during prolonged periods of vasoconstriction “more permanent” actin cytoskeletal structures are formed through actin polymerization pathways. These “more permanent” cytoskeletal structures may be part of the existing cytoskeleton or may represent new cellular processes of the vascular smooth muscle cells that re-elongate and reposition themselves in the arteriolar wall during the remodeling process (see below). Our results suggest that in the initial stages of the inward remodeling process the establishment of these structures does not allow the vessel to dilate to its previous maximal passive diameter.
The generation of filamentous F-actin occurs via the aggregation of globular G-actin monomers into oligomers that are subsequently elongated to form a polarized filament. During the elongation phase, ATP bound monomers are added to both ends of the growing filament, though the rate of addition is not equal, as the designated plus end adds monomers at a much greater rate than the minus end. Once incorporated into filaments, the actin monomers can undergo nucleotide hydrolysis, thereby increasing the rate at which they disassociate from the filament. This dissociation predominantly occurs at the minus end. As the filament grows, the local concentration of G-actin decreases and the filament achieves a steady state equilibrium in which the rate of actin monomer incorporation at the plus end equals the rate of monomers disassociating from the minus end. Throughout this process a host of proteins interact with G and F-actin to maintain and regulate not only filament assembly and elongation, but also filament stability and disassembly.
The formation of actin dimers and trimers, termed nucleation, appears to be the rate-limiting step in F-actin polymerization. The dissociation constant (Kd) for actin dimers has been estimated to be as high as 4.6 M [72]. To overcome this kinetic barrier, cells utilize a number of actin regulators to promote nucleation. To date, three main classes of nucleators have been described in the literature: the Arp2/3 complex, formins and Spire. The activity of the nucleators is controlled by nucleation promoting factors (NPFs). It is well established that in response to vasoconstrictor stimuli actin polymerization is required for maximum force generation as well as for maintenance of constriction in vascular smooth muscle cells (for review see [28]). It is less clear which nucleator (or combination of nucleators) mediates actin polymerization during constriction of resistance arteries. However, the Arp 2/3 complex has been implicated in the process. The NPF, neuronal Wiskott-Aldrich Syndrome Protein (N-WASp), activates the Arp 2/3 complex and promotes nucleation and actin polymerization. In rat mesenteric arteries exposed to phenylephrine, inhibition of N-WASp association with the Arp 2/3 complex decreases the extent of constriction and dampens the increase in the ratio of F- to G-actin (an indicator of actin polymerization) [3]. It has also been shown that the Arp2/3 complex is a component of adhesion complexes that are formed following activation of integrins [24]. Integrins are transmembrane receptor proteins that link the extracellular matrix (ECM) to the cytoskeleton and, in addition to other functions, facilitate cell motility by regulating actin polymerization at adhesion sites [12]. They also play a role in vascular remodeling, as inhibition of αV integrins blocked inward eutrophic remodeling in rat resistance vessels [32]. Interestingly, formins have also been identified in purified adhesion complexes that promote actin polymerization [15]. However, it is not clear what effect, if any, they have on actin polymerization in vascular smooth muscle cells in response to contractile stimulation.
In addition to assembly, actin filament disassembly is also highly regulated. Cofilin-1 is a member of the Actin-depolymerizing factor/cofilin family and, when active, is able to bind adenosine diphosphate subunits in F-actin and sever actin filaments [54]. Paradoxically, cofilin’s depolymerizing effects are concentration dependent. At relatively high concentrations, the severing activity of cofilin promotes actin nucleation by increasing the availability of free monomers to oligomerize. In addition, it is postulated that direct binding of cofilin with actin dimers promotes their stabilization, thereby decreasing the rate of dimer disassociation [2]. Cofilin’s ability to bind to actin is inhibited by its phosphorylation at position Ser3 by Lim Kinase [84]. Interestingly, the signaling pathways of numerous vasoconstrictor agonists lead to Lim Kinase activation, as Rho associated kinase phosphorylates and activates Lim Kinase (Fig. 2) [61]. It remains to be determined what role cofilin and Lim kinase play in vasoconstriction of resistance vessels, and the subsequent development of inward remodeling. Since actin polymerization is required for inducing inward remodeling in resistance vessels, we are intrigued by the possibility that Lim kinase could potentially regulate the process by inactivating cofilin, thereby shifting the actin dynamics towards increased polymerization.
MATRIX METALLOPROTEINASES, INWARD REMODELING AND THE CYTOSKELETON
Inward remodeling induced ex vivo by prolonged vasoconstriction of isolated arterioles or in vivo in animal models of hypertension has been associated with the production of ROS and MMP activity [16, 17, 49]. MMP activity is for the most part associated with extracellular matrix degradation, but there are a number of ways by which MMPs may modulate cytoskeletal structures. The degradation of extracellular matrix structures creates protein fragments with exposed cryptic sites that activate integrins [48]. Integrin activation in turn activates intracellular signals that induce cytoskeletal modifications. Interestingly, the activation of specific integrins is also required for the inward eutrophic remodeling of resistance arteries observed in the Ren2 rat model of hypertension [32]. Additional pathways by which MMPs can induce cytoskeletal modifications include the transactivation the epidermal growth factor receptor as it occurs upon stimulation with vasoconstrictor agonists such as norepinephrine and angiotensin II [55, 82]. The formation of vasoactive compounds such as the vasoconstrictor fragments of endothelin created by the cleavage of big endothelin-1 [27], is another mechanism by which MMPs could induce cytoskeletal modifications and arteriolar inward remodeling. In isolated arterioles we previously showed that prolonged stimulation with norepinephrine and angiotensin II resulted in an increased expression of MMP-2, and an increased level of gelatinolytic activity that was dependent on the production of ROS [49]. Furthermore, we showed that broad MMP inhibition did not affect the production of ROS but prevented the remodeling induced by prolonged vasoconstriction. In contrast, inhibition of ROS prevented both the activation of MMPs and the inward remodeling, suggesting that ROS-dependent activation of MMPs is involved in the development of the remodeling process [49].
INWARD REMODELING, TRANSGLUTAMINASE ACTIVITY AND THE ACTIN CYTOSKELETON
The role of transglutaminase activity on the inward remodeling process of resistance arteries was first demonstrated in a seminal study by Bakker et al. [4]. In that study the authors showed that the inward remodeling induced in vivo by low flow or ex vivo by prolonged exposure to endothelin-1 could be blocked with inhibitors of TG2 activity [4]. They also showed that exogenous application of TG2 or its increased expression in response to retinoic acid exposure also induced inward remodeling in isolated resistance arteries [4]. The transglutaminases are a family of enzymes that promote transamidation, covalently linking a lysine from one protein with the glutamine of another under high calcium conditions such as those found extracellularly [7]. Consequently, the role of TG2 on vascular remodeling has been shown and presumed to include extracellular matrix crosslinking [4, 80]. The role of TG2 on remodeling of the vascular extracellular matrix has also been shown to include vascular calcification in conduit arteries [18, 37]. However, in addition to the well-known extracellular matrix cross-linking functions of TG2, this enzyme is known to contribute to and catalyze other reactions that impact cytoskeletal structures [58]. For example, TG2 has the capability to function as a G-protein, with the potential triggering of pathways that stimulate the activity of small GTPases such as Rho and its downstream effector ROCK leading to the remodeling of the actin cytoskeleton via mechanisms such as the polymerization of actin, the stabilization of existent actin fibers and the inhibition of actin depolymerization [58]. The activation of TG2 at the cell membrane and its association with integrin receptors also has the potential to cluster cell adhesion sites and initiate integrin dependent outside in signaling pathways that lead to cytoskeletal remodeling [7, 48]. The association that exists between TG2 and intracellular stress fibers suggests that TG2 may also help in the formation and stabilization of those fibers. Therefore the role of the transglutaminases on the inward remodeling process of resistance arteries needs to be further investigated to determine their potential participation on the initial stages of the process, which we have shown includes cytoskeletal modifications, and on the specific extracellular matrix changes TG2 causes on inwardly remodeled vessels.
RHO, RAC, AND THE ROLE OF SMOOTH MUSCLE CELL MOTILITY IN ARTERIOLAR REMODELING
The majority of smooth muscle cells within the vessel wall are found in a differentiated state called the contractile phenotype. This differentiated state is characterized by low proliferative capabilities, specific cellular morphologies (i.e., elongated and spindle shaped) and by the expression of contractile molecular markers such as smoothelin, α-smooth muscle actin and smooth muscle-myosin heavy chain. During the remodeling of large conduit vessels (e.g., aorta, carotid artery) encountered in vascular injury, hypertension and atherosclerosis, the contractile smooth muscle cells undergo a process of dedifferentiation towards a synthetic phenotype. An important characteristic of the synthetic phenotype is that the smooth muscle cells acquire the capacity to proliferate and migrate in response to extracellular stimuli such as angiotensin II, norepinephrine, and extracellular matrix fragments. In large conduit arteries, vascular smooth muscle cell migratory behavior has been documented both in vitro and in vivo [11, 36, 39]. However the role of smooth muscle cell migration in the microcirculation has not been investigated. We have previously reported that repositioning of smooth muscle cells from the medial layer of resistance arteries is one of the mechanisms associated with inward eutrophic remodeling [46]. We found that after only four hours of exposure to vasoconstrictor agonists, the vessels manifested structural changes consistent with inward remodeling [45, 46, 49, 74]. Using multiphoton microscopy, it was determined that the reduction in luminal diameter was associated with the rearrangement of a number of smooth muscle cells relative to each other. These smooth muscle cells relengthened and increased their overlapping along their longitudinal axis during the continual exposure to contractile agonists. As mentioned above, in this type of remodeling the cross-sectional area of the vessel wall does not change, while the passive luminal diameter is reduced. This suggests that the reduction in luminal diameter is accomplished by the rearrangement of the same amount of wall material, including smooth muscle cells, around a smaller vascular lumen. Our results suggest that a number of smooth muscle cells reposition around a smaller lumen resulting in luminal narrowing and impaired capacity for dilation. Although the cytoskeleton was not specifically investigated in that study, the repositioning of the smooth muscle cells likely requires a coordinated rearrangement of cytoskeletal structures in addition to the acto-myosin cross bridge cycling associated with smooth muscle cell contraction. Furthermore, the cytoskeletal reorganization is likely coordinated with changes in cell-matrix adhesions [33], as suggested by studies indicating that integrin associated mechanisms are required for the inward eutrophic remodeling process to occur [31, 32].
Actin cytoskeleton reorganization and dynamics are critical for smooth muscle cell movement and are associated with changes in cell shape and polarity [29]. The cell movement is coordinated by the key regulators of the cytoskeleton, Rho, Rac and Cdc42. These small GTPases control numerous aspects of actin-filament turnover and assembly [38, 65]. Each GTPase can induce different changes in the cytoskeleton. For example, Rho activation results in the formation of stress fibers which contain acto-myosin filaments and are associated with focal adhesion complexes [66]. Also, through its downstream target Rho kinase (ROCK), Rho is associated with calcium sensitization [73]. In comparison, Rac activation induces the formation of membrane ruffles and lamellipodia leading to reorganization of the cytoskeleton [56]. Cdc42 has been shown to induce the formation of filopodia. Cdc42 activation, induced by exposure to contractile agonists, has also been shown to control actin polymerization and active tension development in tracheal smooth muscle cells [40, 57]. In a recent study from our laboratory we investigated the role of the small GTPases in the inward remodeling process of resistance arteries [74]. Using isolated vessels, we determined that ROCK or Rac-1 inhibition with the pharmacological agents Y27632 or NSC23766, respectively, prevented the inward eutrophic remodeling induced by prolonged exposure to contractile agonists. ROCK inhibition prevented the maintenance of agonist induced constriction and inward remodeling, while Rac-1 inhibition prevented the remodeling but allowed prolonged vasoconstriction to be maintained. We also found that ROCK inhibition interfered with the maintenance of basal tone. This is consistent with studies showing that in pressurized vessels Rho-ROCK pathways maintain calcium sensitivity in the absence of vasoconstrictor agonists [41, 71, 81], and with studies showing that ROCK affects actin polymerization [22]. That the early remodeling process was dependent on actin cytoskeletal modification was corroborated by the observation that actin fiber disruption reversed the inward remodeling caused by prolonged vasoconstriction. However, the pathways involved in inward remodeling that are more likely to be affected by the pharmacological blockade of ROCK or Rac-1 remain to be experimentally determined.
Taken together our studies suggest that the early phases of constriction-induced inward remodeling involve the dynamic reorganization of smooth muscle cells around a smaller lumen. This rearrangement appears to be dependent on the actin cytoskeletal reorganization orchestrated by the small GTPases Rho and Rac. A possible mechanism of remodeling would involve two concomitant processes. First, once the smooth cells are exposed to contractile agonists they begin to constrict and continue to maintain the constriction over time. This process is dependent mainly on the Rho-ROCK pathway. In parallel with this process a number of smooth muscle cells relengthen, readjust their position within the media and increase their cellular overlap. This latter process might be locally and temporally coordinated by the interplay between Rho, Rac and Cdc42 pathways. During this process of readjustment, the cells might extend lamellipodia and filopodia coordinated by Rac and possibly Cdc42, while simultaneously forming stress fibers through Rho activity. This model is consistent with our findings that ROCK inhibition prevents both the maintenance of constriction and remodeling, whereas Rac inhibition only prevents the remodeling without affecting acute or prolonged constriction. However, the validity of this model needs to be confirmed experimentally.
CONCLUSIONS AND FUTURE DIRECTIONS
A substantial number of studies provide strong evidence that a number of cardiovascular diseases are associated with structural changes in resistance arteries, with inward eutrophic remodeling being the most prevalent in hypertension. Results from recent studies have associated the development of inward eutrophic remodeling with processes that involve prolonged vasoconstriction, actin polymerization, transglutaminase activity and ROS-dependent activation of MMPs. However, a complete pathway that connects all these phenomena, and provides a full temporal and mechanistic description of the remodeling process is still warranted. Additional studies should therefore investigate the temporal associations and interactions that link prolonged vasoconstriction with actin polymerization pathways, the oxidative state of cells, activation of MMPs and transglutaminase activity. Elucidation of these associations and interactions should provide a clearer view of the mechanisms that control inward eutrophic remodeling and consequently present targets for therapeutic intervention and reduction of the life threatening events associated with resistance vessel remodeling.
ACKNOWLEDGMENTS
Authors are supported by the National Heart, Lung, and Blood Institute, Grant HL-088105 to LAM-L.
Abbreviations used
- CSA
cross-sectional area
- ROS
reactive oxygen species
- MMP
matrix metalloproteinases
- TG2
tissue type transglutaminase
- ROCK
Rho kinase
REFERENCES
- 1.Adler KB, Krill J, Alberghini TV, Evans JN. Effect of cytochalasin D on smooth muscle contraction. Cell motility. 1983;3:545–551. doi: 10.1002/cm.970030521. [DOI] [PubMed] [Google Scholar]
- 2.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96:119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 5.Bakker EN, Buus CL, VanBavel E, Mulvany MJ. Activation of resistance arteries with endothelin-1: from vasoconstriction to functional adaptation and remodeling. J Vasc Res. 2004;41:174–182. doi: 10.1159/000077288. [DOI] [PubMed] [Google Scholar]
- 6.Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res. 2006;99:86–92. doi: 10.1161/01.RES.0000229657.83816.a7. [DOI] [PubMed] [Google Scholar]
- 7.Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- 8.Baumbach GL, Dobrin PB, Hart MN, Heistad DD. Mechanics of cerebral arterioles in hypertensive rats. Circ Res. 1988;62:56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- 10.Baumbach GL, Walmsley JG, Hart MN. Composition and mechanics of cerebral arterioles in hypertensive rats. Am J Pathol. 1988;133:464–471. [PMC free article] [PubMed] [Google Scholar]
- 11.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. American Journal of Pathology. 2002;160:1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blystone SD. Integrating an integrin: a direct route to actin. Biochim Biophys Acta. 2004;1692:47–54. doi: 10.1016/j.bbamcr.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, Gonzalez MC, McGrath JC, Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. The Journal of physiology. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briones AM, Rodriguez-Criado N, Hernanz R, Garcia-Redondo AB, Rodrigues-Diez RR, Alonso MJ, Egido J, Ruiz-Ortega M, Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54:142–149. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- 15.Butler B, Gao C, Mersich AT, Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr Biol. 2006;16:242–251. doi: 10.1016/j.cub.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Castro MM, Rizzi E, Ceron CS, Guimaraes DA, Rodrigues GJ, Bendhack LM, Gerlach RF, Tanus-Santos JE. Doxycycline ameliorates 2K-1C hypertension-induced vascular dysfunction in rats by attenuating oxidative stress and improving nitric oxide bioavailability. Nitric Oxide. 2012;26:162–168. doi: 10.1016/j.niox.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, Tanus-Santos JE. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free radical biology & medicine. 2009;46:1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen NX, O'Neill K, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Transglutaminase 2 accelerates vascular calcification in chronic kidney disease. American journal of nephrology. 2013;37:191–198. doi: 10.1159/000347031. [DOI] [PubMed] [Google Scholar]
- 19.Chillon JM, Baumbach GL. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in Wistar-Kyoto rats. J Hypertens. 2004;22:529–534. doi: 10.1097/00004872-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- 21.Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, Martinez-Lemus LA, Dora KA, Yang Y, Davis MJ, Pohl U, Meininger GA, Hill MA. Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2889–2896. doi: 10.1161/ATVBAHA.111.236570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corteling RL, Brett SE, Yin H, Zheng X-L, Walsh MP, Welsh DG. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H440–H447. doi: 10.1152/ajpheart.01374.2006. [DOI] [PubMed] [Google Scholar]
- 23.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS letters. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 24.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–891. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobrin PB. Mechanical properties of arteries. Physiological Reviews. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- 26.Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27:317–324. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 28.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall A. Rho GTPases and the Actin Cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 30.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 31.Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling, and hypertension. Hypertension. 2007;49:1–4. doi: 10.1161/01.HYP.0000252753.63224.3b. [DOI] [PubMed] [Google Scholar]
- 32.Heerkens EH, Shaw L, Ryding A, Brooker G, Mullins JJ, Austin C, Ohanian V, Heagerty AM. alphaV integrins are necessary for eutrophic inward remodeling of small arteries in hypertension. Hypertension. 2006;47:281–287. doi: 10.1161/01.HYP.0000198428.45132.02. [DOI] [PubMed] [Google Scholar]
- 33.Hong Z, Sun Z, Li Z, Mesquitta WT, Trzeciakowski JP, Meininger GA. Coordination of fibronectin adhesion with contraction and relaxation in microvascular smooth muscle. Cardiovasc Res. 2012;96:73–80. doi: 10.1093/cvr/cvs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Intengan HD, Deng LY, Li JS, Schiffrin EL. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension. 1999;33:569–574. doi: 10.1161/01.hyp.33.1.569. [DOI] [PubMed] [Google Scholar]
- 35.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 36.Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:e35–e44. doi: 10.1161/ATVBAHA.111.225623. [DOI] [PubMed] [Google Scholar]
- 37.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaibuchi K, Kuroda S, Amano M. Regulation of the Cytoskeleton and Cell Adhesion by the Rho Family GTPases in Mammalian Cells. Annual Review of Biochemistry. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 39.Kohno M, Ohmori K, Nozaki S, Mizushige K, Yasunari K, Kano H, Minami M, Yoshikawa J. Effects of valsartan on angiotensin II-induced migration of human coronary artery smooth muscle cells. Hypertension Research. 2000;23:677–681. doi: 10.1291/hypres.23.677. [DOI] [PubMed] [Google Scholar]
- 40.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagaud G, Gaudreault N, Moore EDW, Breemen Cv, Laher I. Pressure-dependent myogenic constriction of cerebral arteries occurs independently of voltage-dependent activation. American Journal of Physiology - Heart and Circulatory Physiology. 2002;283:H2187–H2195. doi: 10.1152/ajpheart.00554.2002. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. Medical & biological engineering & computing. 2008;46:469–478. doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loufrani L, Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Medical & biological engineering & computing. 2008;46:451–460. doi: 10.1007/s11517-008-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Lemus LA. The dynamic structure of arterioles. Basic Clin Pharmacol Toxicol. 2012;110:5–11. doi: 10.1111/j.1742-7843.2011.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez-Lemus LA. Persistent Agonist-Induced Vasoconstriction Is Not Required for Angiotensin II to Mediate Inward Remodeling of Isolated Arterioles with Myogenic Tone. J Vasc Res. 2008;45:211–221. doi: 10.1159/000112513. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. Faseb J. 2004;18:708–710. doi: 10.1096/fj.03-0634fje. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Lemus LA, Zhao G, Galinanes EL, Boone M. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. American journal of physiology Heart and circulatory physiology. 2011;300:H2005–2015. doi: 10.1152/ajpheart.01066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine. 3:e442–2006. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathiassen ON, Buus NH, Larsen ML, Mulvany MJ, Christensen KL. Small artery structure adapts to vasodilatation rather than to blood pressure during antihypertensive treatment. J Hypertens. 2007;25:1027–1034. doi: 10.1097/HJH.0b013e3280acac75. [DOI] [PubMed] [Google Scholar]
- 52.Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens. 2007;25:1021–1026. doi: 10.1097/HJH.0b013e32805bf8ed. [DOI] [PubMed] [Google Scholar]
- 53.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol 519 Pt. 1999;3:829–840. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moriyama K, Yahara I. Two activities of cofilin, severing and accelerating directional depolymerization of actin filaments, are affected differentially by mutations around the actin-binding helix. Embo J. 1999;18:6752–6761. doi: 10.1093/emboj/18.23.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagareddy PR, Chow FL, Hao L, Wang X, Nishimura T, MacLeod KM, McNeill JH, Fernandez-Patron C. Maintenance of adrenergic vascular tone by MMP transactivation of the EGFR requires PI3K and mitochondrial ATP synthesis. Cardiovasc Res. 2009;84:368–377. doi: 10.1093/cvr/cvp230. [DOI] [PubMed] [Google Scholar]
- 56.Nobes CD, Hall A. Rho GTPases Control Polarity, Protrusion, and Adhesion during Cell Movement. The Journal of Cell Biology. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 58.Nurminskaya MV, Belkin AM. Cellular functions of tissue transglutaminase. International review of cell and molecular biology. 2012;294:1–97. doi: 10.1016/B978-0-12-394305-7.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obara K, Yabu H. Effect of cytochalasin B on intestinal smooth muscle cells. Eur J Pharmacol. 1994;255:139–147. doi: 10.1016/0014-2999(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 60.Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;46:93–99. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- 61.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 62.Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol. 2007;293:C993–1002. doi: 10.1152/ajpcell.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhodin JAG. Architecture of the Vessel Wall. In: Compr Physiol 2011, Supplement 7: Handbook of Physiology, The Cardiovascular System, Vascular Smooth Muscle: 1-31.First published in print 1980. doi: 10.1002/cphy.cp020201. [Google Scholar]
- 64.Rhodin JAG. The ultrastructure of mammalian arterioles and precapillary sphincters. Journal of Ultrasructure Research. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 65.Ridley AJ. Rho family proteins and regulation of the actin cytoskeleton. Progress in molecular and subcellular biology. 1999;22:1–22. doi: 10.1007/978-3-642-58591-3_1. [DOI] [PubMed] [Google Scholar]
- 66.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 67.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 68.Saito SY, Hori M, Ozaki H, Karaki H. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 1996;32:51–60. doi: 10.1540/jsmr.32.51. [DOI] [PubMed] [Google Scholar]
- 69.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17:1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 70.Schnittler HJ, Schneider SW, Raifer H, Luo F, Dieterich P, Just I, Aktories K. Role of actin filaments in endothelial cell-cell adhesion and membrane stability under fluid shear stress. Pflugers Archiv : European journal of physiology. 2001;442:675–687. doi: 10.1007/s004240100589. [DOI] [PubMed] [Google Scholar]
- 71.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. American Journal of Physiology - Heart and Circulatory Physiology. 2002;283:H2288–H2295. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 72.Sept D, McCammon JA. Thermodynamics and kinetics of actin filament nucleation. Biophys J. 2001;81:667–674. doi: 10.1016/S0006-3495(01)75731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinases, and Myosin Phosphatase. Physiological Reviews. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 74.Staiculescu MC, Galinanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res. 2013;98:428–436. doi: 10.1093/cvr/cvt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su Y, Edwards-Bennett S, Bubb MR, Block ER. Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. American journal of physiology Cell physiology. 2003;284:C1542–1549. doi: 10.1152/ajpcell.00248.2002. [DOI] [PubMed] [Google Scholar]
- 76.Su Y, Zharikov SI, Block ER. Microtubule-active agents modify nitric oxide production in pulmonary artery endothelial cells. American journal of physiology Lung cellular and molecular physiology. 2002;282:L1183–1189. doi: 10.1152/ajplung.00388.2001. [DOI] [PubMed] [Google Scholar]
- 77.Tseng S, Kim R, Kim T, Morgan KG, Hai CM. F-actin disruption attenuates agonist-induced [Ca2+], myosin phosphorylation, and force in smooth muscle. Am J Physiol. 1997;272:C1960–1967. doi: 10.1152/ajpcell.1997.272.6.C1960. [DOI] [PubMed] [Google Scholar]
- 78.Ueki Y, Uda Y, Sakamoto N, Sato M. Measurements of strain on single stress fibers in living endothelial cells induced by fluid shear stress. Biochemical and biophysical research communications. 2010;395:441–446. doi: 10.1016/j.bbrc.2010.04.051. [DOI] [PubMed] [Google Scholar]
- 79.Van Den Akker J, Schoorl MJC, Bakker ENTP, Vanbavel E. Small artery remodeling: Current concepts and questions. Journal of Vascular Research. 2010;47:183–202. doi: 10.1159/000255962. [DOI] [PubMed] [Google Scholar]
- 80.van den Akker J, VanBavel E, van Geel R, Matlung HL, Guvenc Tuna B, Janssen GM, van Veelen PA, Boelens WC, De Mey JG, Bakker EN. The redox state of transglutaminase 2 controls arterial remodeling. PLoS ONE. 2011;6:e23067. doi: 10.1371/journal.pone.0023067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.VanBavel E, van der Meulen E, Spaan J. Role of Rho-associated protein kinase in tone and calcium sensitivity of cannulated rat mesenteric small arteries. Experimental Physiology. 2001;86:585–592. doi: 10.1113/eph8602217. [DOI] [PubMed] [Google Scholar]
- 82.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 83.Yamin R, Morgan KG. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J Physiol. 2012;590:4145–4154. doi: 10.1113/jphysiol.2012.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 85.Zhou MS, Jaimes EA, Raij L. Vascular but not cardiac remodeling is associated with superoxide production in angiotensin II hypertension. J Hypertens. 2005;23:1737–1743. doi: 10.1097/01.hjh.0000179513.71018.09. [DOI] [PubMed] [Google Scholar]