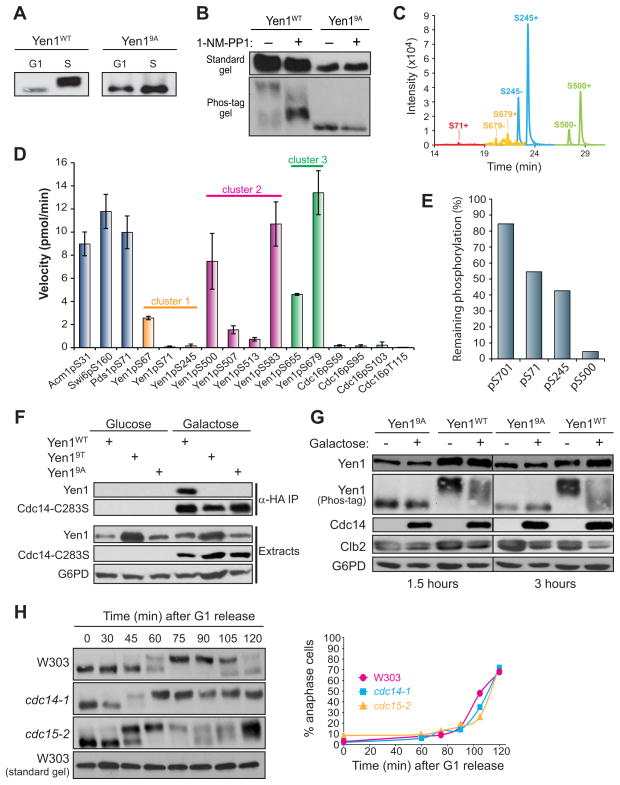
Figure 2. Yen1 is an in vivo substrate of Cdk and Cdc14.
A) Anti-protein A immunoblots from Phos-tag SDS-PAGE. Extracts from cells arrested in G1 or S and expressing integrated YEN1-TAP or yen19A-TAP from PYEN1 were compared. B) Same as A except asynchronous cdc28-as1 cells were treated with 20 μM 1-NM-PP1 or an equal volume of DMSO for 30 min. Immunoblots using standard and Phos-tag gels are shown to emphasize the phosphorylation-dependence of the mobility shift. C) Ion chromatograms for Yen1 tryptic peptides containing the indicated Cdk phosphorylation sites in both unmodified (“−“) and phosphorylated (“+”) forms detected by LC-MS. Identity of the peak in each case was verified by product ion spectra (Fig. S2). Unmodified S71 peptide was undetectable D) Activity of Cdc14 towards the indicated phosphopeptide substrates was measured under identical steady state conditions. Data are the average of three trials and error bars are standard deviations. Peptide sequences shown in Table S4. E) Yen1-3FLAG purified from yeast was treated with 250 nM recombinant Cdc14 for 10 min and analyzed by LC-MS. Ion signals for peptides phosphorylated at the indicated residues were integrated and percent phosphorylation remaining relative to untreated Yen1-3FLAG was calculated using unmodified peptides for normalization. F) Co-IP of the substrate trap 3HA-Cdc14-C283S expressed from PGAL1 and Yen1-TAP, Yen19A-TAP, or Yen19T-TAP expressed from PYEN1 in S phase cells. 3HA-Cdc14-C283S was isolated on anti-HA affinity resin and co-purification of Yen1 variants monitored by immunoblotting. G6PD is a loading control. G) 3HA-Cdc14 was ectopically overexpressed from PGAL1 for either 1.5 or 3 hours in S phase cells expressing Yen1-TAP or Yen19A-TAP from PYEN1. H) W303, cdc14-1, and cdc15-2 strains, each expressing Yen1-TAP from PYEN1 were arrested at 23 °C in G1, then released into fresh medium at 37 °C. Cells were harvested at the indicated times for analysis by Phos-tag immunoblotting and nuclear staining. The graph shows similar cell cycle kinetics based on percentage of anaphase cells (large-budded with two DNA masses).