Abstract
An efficient hairy root induction system for an important endangered medicinal plant, Dracocephalum kotschyi, was developed through Agrobacterium rhizogenes-mediated transformation by modifying the co-cultivation medium using five bacterial strains, A4, ATCC15834, LBA9402, MSU440, and A13 (MAFF-02-10266). A drastic increase in transformation frequency was observed when a Murashige and Skoog medium lacking NH4NO3 KH2PO4, KNO3 and CaCl2 was used, resulting in hairy root induction frequencies of 52.3 %, 69.6 %, 48.6 %, 89.0 %, and 80.0 % by A4, A13, LBA9402, MSU440, and ATCC15834 strains, respectively. For shoot induction, hairy roots and unorganized tumors induced by strain ATCC15834 were placed on an MS media supplemented with 0.1, 0.25, 0.5, and 1 mg/l BA plus 0.1 mg/l NAA. The high frequency of shoot regeneration and number of shoot were obtained in the medium containing 0.25 mg/l BA and 0.1 mg/l NAA. Root induction occurred from the base of regenerated shoots on the MS medium supplemented with 0.5 mg/l IBA after 10 days.
Keywords: Agrobacterium strains, Co-cultivation medium, Dracocephalum kotschyi, Hairy root, Regeneration
Introduction
Dracocephalum kotschyi (belonging to the Labiatae family) is an important endangered endemic medicinal plant species found in Isfahan and the Alborz Mountains of Iran (Rechinger 1982). The propagation D. kotschyi by seed is restricted because of strong seed dormancy. In traditional medicine, D. kotschyi is used for stomach and liver disorders because of its antispasmodic and analgesic properties (Jahaniani et al. 2005). It is also used for its antihyperlipidermic effects and immune modulatory effects (on rheumatoid arthritis) (Amirghofran et al. 2000). This plant inhibits the multiplication of tumor cells; thus it has been used in the development of drugs effective against cancer (Cordell et al. 1991). An anticancer drug (Spinal-Z) introduced by Iranian researchers, which has a specific effect against leukemia, is extracted from D. kotschyi leaves and Peganum harmala seeds (Sobhani et al. 2002; Jahaniani et al. 2005).
Hairy root is a plant disease induced by A. rhizogenes. Root loci (rol) genes harbored by the root inducing (Ri) plasmid of A. rhizogenes are integrated into the nuclear genome of the host plant, inducing hairy root. Several factors affect the rate of A. rhizogenes mediated transformation in different plants, including acetosyringone, A. rhizogenes strains, and modified MS compounds (Sharafi et al 2013b).
Hairy root-derived plants are usually real transgenic (Maknight et al. 1987). Putative transgenic regenerated plants from hairy roots could be formed directly or indirectly (via callus). He-Ping et al. (2011) successfully reported adventitious shoot formation from Pogostemon cablin hairy roots. Also, regenerated shoots from the calli of hairy roots were formed in Malus baccata (Wu et al. 2012).
In this study, an efficient protocol for hairy root induction of D. kotschyi and plant regeneration from hairy roots was established. The improved transformation was achieved by modifying several chemical compounds in co-cultivation media and selection of suitable strains of A. rhizogenes. Regeneration system was also successfully optimized from hairy roots. To the best of our knowledge, this is the first report of hairy root induction and regeneration of medicinal plant D. kotschyi.
Materials and methods
Seed sterilization and germination
Seeds of D. kotschyi (collected from Isfahan province, Iran) were surface sterilized by immersing with 70 % (v/v) ethanol for 2 min and 3 % (v/v) sodium hypochlorite solution for 15 min and then washed five times using sterilized water. The seeds were placed on 0.7 % basal MS-agar plates (Murashige and Skoog 1962). The medium was adjusted to pH 5.8 before adding agar and was sterilized by autoclaving. The seeds were germinated at 25 °C under a 16/8 h (day/night) photoperiod in a culture room.
Preparation of A. rhizogenes strains
Five strains of A. rhizogenes were used: A4, ATCC15834, LBA9402, MSU440 (agropine type strains) and a mikimopin producing strain A13 (MAFF-02-10266). All the strains were provided by the bank of microbes at the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. The A. rhizogenes strains were cultured in liquid Luria-Bertani medium containing 50 mg/l rifampicin, 10 g/l tryptone, 5 mg/l yeast extract and 10 mg/l NaCl, pH 7.2 to an optical density of 0.6, at 28 °C, 160 rpm on a shaker incubator. The bacteria were pelleted by centrifugation for 15 min at 3,000 rpm and resuspended to a cell density of OD600 0.8 in liquid inoculation medium, consisted of MS salts and vitamins along with 50 mg/l sucrose and 100 μM acetosyringone (designated as Medium 1). This medium was subsequently modified by removing the following compounds: KH2PO4 (Medium 2); KH2PO4, CaCl2 (Medium 3); KH2PO4, NH4NO3, KNO3 (Medium 4); KH2PO4, NH4NO3, KNO3, CaCl2 (Medium 5) based on our previous studies (Azadi et al. 2010; Sharafi et al. 2013a, c).
Co-cultivation and induction of hairy root culture
Leaf explants from 4-week old D. kotschyi seedlings were used for co-cultivation with different strains of A. rhizogenes. The explants were randomly wounded using sterile needle and inoculated with different bacterial strains (ATCC 15834, A4, LBA9402, MSU440, A13) for 10 min. The explants were briefly dried with sterile filter paper and then transferred to co-cultivation medium which was the same as the inoculation medium but solidified with agar. After 2 days of co-cultivation, the explants transferred to MS media supplemented with 400 mg/l cefotaxime to eliminate the A. rhizogenes. Control explants were treated similarly but without inoculation with A. rhizogenes.
Shoot regeneration from hairy roots
Hairy roots and tumors induced by strain ATCC15834 were excised into 2–3 cm segments and transferred to regeneration media. MS solid media supplemented with 3 % sucrose, 0.1, 0.25, 0.5 and 1 mg/l BA in companion with 0.1 mg/l NAA, pH 5.8 was used as regeneration medium. After 3 weeks, regenerated shoots (2–2.5 cm) were excised and cultivated on root induction medium (MS medium supplemented with 0.5 mg/l IBA).
Polymerase chain reaction analysis
Total DNA was isolated from hairy root samples, putative transgenic regenerated plants, and control roots using genomic plant DNA extraction kit (iNtRON Biotechnology Co.). Isolated DNA was used in PCR analysis for detecting the rol B gene. The primers designed to amplify rol B were 5′ GCTCTTGCAGTGCTAGATTT3′ and 5′ GAAGGTGCAAGCTACCTCTC3′.
Statistical analysis
The experiments were laid on a completely randomized design (CRD) with six replications and nine explants cultured in each Petri dish. The data collected were subjected to analysis of variance test. The means were compared using Duncan’s multiple range tests. The data expressed as percentage were subjected to arcsine transformation before the statistical analysis.
Results and discussion
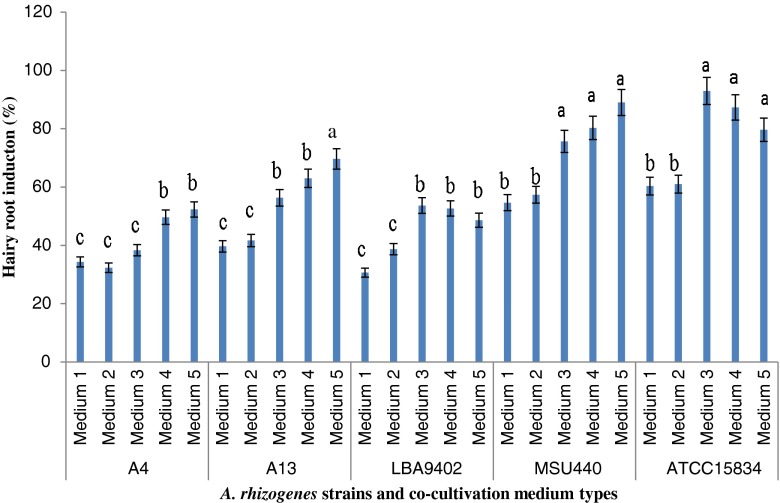
Effect of different co-cultivation media and A. rhizogenes strains
Seeds were germinated for 1 week, and leaf explants from 4-week-old D. kotschyi seedlings were used for co-cultivation. The effect of five A. rhizogenes strains and five co-cultivation media were evaluated on hairy root induction of D. kotschyi leaf explants (Fig. 1a–f). All of the treatments led to hairy root induction. Explants were transferred to fresh media every 2 weeks to avoid browning (Fig. 1g). Tumor induction along with hairy roots was observed using strain ATCC15834 after 5 weeks of inoculation (Fig. 1h). The induced hairy roots were excised and put into an MS medium containing 400 mg/l cefotaxime to eliminate bacteria.
Fig. 1.
A. rhizogenes mediated transformation in D. kotschyi (a) leaf explants (b, c, d, e, f) hairy root induction on explants after 4 weeks with inoculation medium 1, 2, 3, 4, and 5 respectively, using strain A13 (MAFF-02-10266); (g) explant browning (h) induction of tumor in companion with hairy roots after 5 weeks of inoculation using strain ATCC15834 in medium 4
In the full strength MS medium (medium 1), the maximum rate of transformation was observed at 60 % in the ATCC15834 strain (Fig. 2). Previous studies showed that the expression of vir G can be activated by low levels of PO4 and suggested that a shortage of PO4 could be a positive signal to induce the infection of plants (Winans 1990). Removing KH2PO4 from inoculation and co-cultivation media (medium 2), however, had no significant effect on transformation frequency.
Fig. 2.
Effect of different A. rhizogenes strains and co-cultivation media on percentage of hairy root induction in D. kotschyi. The data were obtained as a mean of four replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s multiple range test. Vertical lines represent standard errors
Using medium 3 or 4 (lacking KH2PO4 and CaCl2 or NH4NO3 KH2PO4 and KNO3 respectively) obviously promoted root induction frequency. In medium 5, in which CaCl2 was additionally removed, transformation frequency drastically increased in comparison with the full strength MS medium (medium 1) in all strains and resulted in 52.3 %, 69.6 %, 48.6 %, 89.0 %, and 80.0 % hairy root induction frequency in A4, A13, LBA9402, MSU440, and ATCC15834 strains, respectively (Fig. 2). Montoro et al. (2000) indicated that the removal of CaCl2 significantly improved Hevea brasiliensis transformation efficiency. Similar results were reported in the case of A. tumefaciens in Lilium transformation (Azadi et al. 2011) and hairy root induction of Papaver bracteatum (Sharafi et al. 2013b). Dupre et al. (2000) indicated that to achieve the highest rate of transformation in Ginkgo biloba, the best co-cultivation medium was a medium lacking mineral components.
As the transformation efficiency was drastically increased by removing NH4NO3, KNO3 and CaCl2, our results suggest that most macro-elements in co-cultivation media have inhibitory effects on the A. rhizogenes mediated transformation of D. kotschyi. Cell proliferation of A. rhizogenes around the explants during co-cultivation in media 4 and 5 was highly increased compared to the MS full strength medium. The bacterial overgrowth in these media proved the inhibitory effect of macro-elements on the proliferation of A. rhizogenes during co-cultivation (data not shown).
The roles of several factors related to the ion effect on Agrobacterium mediated transformation have been reported. Some possible reasons for an increased rate of transformation could be the effect of PO4 starvation in activating vir G expression (Winans 1990), the formation of biofilm (Danhorn et al. 2004), the possibility of a Ca+2 inhibitory effect on the expression of virulence genes of bacteria (Flego et al. 1997), and increased proliferation of Agrobacterium in the medium which is lacking some elements (Azadi et al. 2010). Similar reports have shown that the ability and frequency of hairy root induction are influenced by the salt concentration of the MS medium. Wu et al. (2008) reported 80.6 % and 81.5 % rooting efficiency in ½ × MS and ¼ × MS media. The high rate of root formation obtained in low strength MS salt medium in Solidago altissima has also been reported (Inoguchi et al. 2003). More studies on the role of these elements are needed to discover how T-DNA transfer is regulated by mineral compounds.
The ability of A. rhizogenes to infect plant species are strain dependent (Porter 1991; Sharafi et al. 2013b). Among the different strains evaluated for induction of transformed hairy roots in D. kotschyi, the MSU440 and ATCC15834 strains showed the highest rates of hairy root induction in each tested co-cultivation medium (Fig. 2). Tumor induction occurred in all strains with differing frequencies (data not shown). Agriopin-type strains are a split T-DNA consisting of two T-DNA regions, TR and TL. The TR-DNA, which only exists in agropine-type plasmids of A. rhizogenes and controls opine and auxin biosynthesis, is responsible for tumors (Veena and Taylor 2007). Sudha et al. (2012) indicated that tumors might be induced by the effects of endogenous hormones accompanied by a bacterial strain in response to the rol gene products.
Shoot regeneration from hairy root cultures
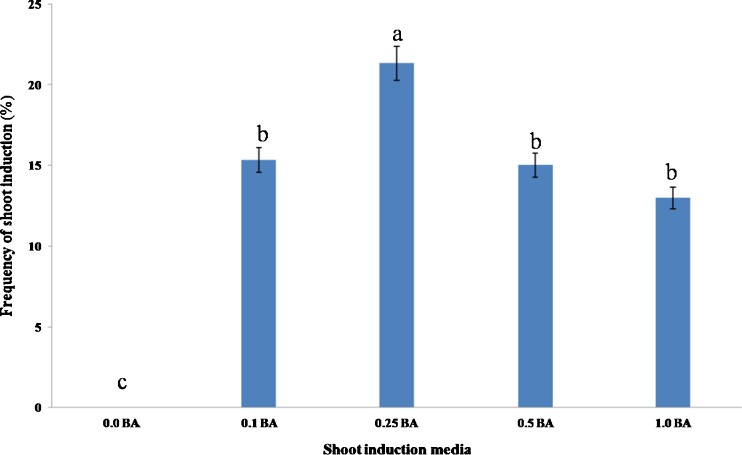
Hairy roots and unorganized tumors induced by strain ATCC15834 were placed on an MS media supplemented with 0.1, 0.25, 0.5 and 1 mg/l BA in companion with 0.1 mg/l NAA for shoot induction. After 2 weeks most hairy root segments induced callus, while the size of tumors increased (Fig. 3a and b). Several small shoot points were formed on the surface of calli from both hairy roots and enlarged tumors (Fig. 3c and d). The number of adventitious shoots increased with time (Fig. 3e) (data not shown). The differentiation rate of adventitious shoots from the calli was significantly affected by the influence of BA. Using 0.25 mg/l BA plus 0.1 mg/l NAA significantly increased the rate of shoot induction (21.3 %) (Fig. 4) and number of shoot per explants (6.0). Although the frequency of adventitious shoot induction (Fig. 4) and the number of shoots (data not shown) decreased in higher levels of BA. In our study no shoot induction was observed in the hormone-free medium which resembles the results of studies on Pogostemon cablin by He-Ping et al. (2011). In contrast, spontaneous regeneration of transformed plants from hairy roots of Plumbago indica (Gangopadhyay et al. 2010) and Centaurium erythraea (Subotic et al. 2009) was reported. Mano and Matsuhashi (1995) reported that horseradish plants can easily induce adventitious shoots from hairy roots without the addition of plant growth regulators. The 2–2.5 cm regenerated shoots were transferred to MS medium supplemented with 0.5 mg/l IBA for rooting. Root induction (80 %) occurred from the base of the shoots in 10 days (Fig. 3f).
Fig. 3.
Adventitious shoot regenerated from hairy roots and unorganized tumors of D. kotschyi (a and b) unorganized tumor and hairy root before adventitious shoot induction; c and d induction of adventitious shoot from hairy root and tumors; e growth of regenerated shoots after 2 weeks from hairy root (f) rooting of shoots originated from hairy root
Fig. 4.
Effect of different concentration of BA in MS medium supplemented with 0.1 mg/l NAA on frequency of adventitious shoot induction in D. kotschyi hairy roots induced by strain ATCC15834. The data were obtained as a mean of four replications. The different letters denote a statistically significant difference at P ≤ 0.05, as determined by Duncan’s multiple range test. Vertical lines represent standard errors
Confirmation of rol B gene in hairy roots and regenerated plants
The PCR analysis of hairy roots and regenerated plants resulted in the amplification of expected fragment similar to that found in the positive control, while there was no amplification in the DNA isolated from normal roots (Fig. 5).
Fig. 5.
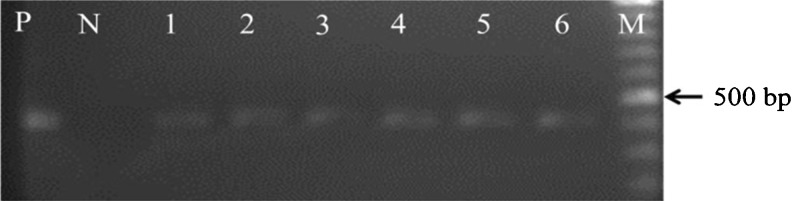
Representative PCR analysis for detection of the rol B gene in hairy roots and regenerated D. kotschyi plant. Lane M molecular size marker (ladder Mix fermentas), lane N an untransformed root as negative control, lane P positive control (pRiATCC15834), lanes 1 – 5 hairy root lines; lane 6 regenerated D. kotschyi from hairy root
This study, for the first time, established a reliable protocol for hairy root induction and plant regeneration of the important medicinal plant D. kotschyi. The propagation of rare medicinal plant D. kotschyi by seed is restricted because of strong seed dormancy, therefore our plant regeneration system will facilitate the clonal propagation. Hairy root offers suitable prospects for the production of valuable compounds and is considered as a green factory. Further studies are in progress to commercialize large scale production of an anticancer compound from this plant by designing a cost effective bioreactor. Moreover, the use of fast-growing hairy roots could have immense potential in investigating the molecular regulation of genes encoding anticancer biosynthetic enzymes.
Acknowledgments
This work was supported by Novin Giti Gene Biotech. Co. Biotechnology Incubator Center of National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.
Abbreviations
- BA
6- Benzyladenine
- NAA
α-Naphthalene Acetic Acid
- IBA
Indol-3- Butyric Acid
Contributor Information
Ali Sharafi, Email: sharafi.a@gmail.com.
Haleh Hashemi Sohi, Email: halehsohi@gmail.com.
Pejman Azadi, Email: azadip22@gmail.com.
Ata Allah Sharafi, Phone: +98-21-44580436, FAX: +98-21-44580436, Email: novingenes@gmail.com.
References
- Amirghofran Z, Azadbakht M, Karimi MH. Evaluation of the immune modulatory effects of five herbal plants. J Ethnopharmacol. 2000;72:167–172. doi: 10.1016/S0378-8741(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Azadi P, Chin DP, Kuroda K, Khan RS, Mii M. Macro elements in inoculation and co-cultivation medium strongly affect the efficiency of Agrobacterium-mediated transformation in Lilium. Plant Cell Tissue Organ Cult. 2010;101:201–209. doi: 10.1007/s11240-010-9677-9. [DOI] [Google Scholar]
- Azadi P, Valentaine Otange N, Supaporn H, Khan RS, Chin DP, Nakamura I, Mii M. Increased resistance to cucumber mosaic virus (CMV) in Lilium transformed with a defective CMV replicase gene. Biotechnol Lett. 2011;33:1249–1255. doi: 10.1007/s10529-011-0550-7. [DOI] [PubMed] [Google Scholar]
- Cordell GA, Beecher CWW, Pezzuuto JM. Can ethnopharmacology contributes to the development of new anticancer drugs. J Ethnopharmacol. 1991;32:117–133. doi: 10.1016/0378-8741(91)90110-Y. [DOI] [PubMed] [Google Scholar]
- Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C. Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR–PhoB regulatory system. J Bacteriol. 2004;186:4492–4501. doi: 10.1128/JB.186.14.4492-4501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre P, Lacoux Y, Neutelings G, Mattar-Lavrain D, Fliniaux MA, David A, Jacquin-Dubreuil A. Genetic transformation of Ginkgo biloba by Agrobacterium tumefaciens. Physiol Plant. 2000;108:413–419. doi: 10.1034/j.1399-3054.2000.t01-1-100411.x. [DOI] [Google Scholar]
- Flego D, Pirhonen M, Saarilahti H, Palva TK, Palva ET. Control of virulence gene expression by plant calcium in the phytopathogen Erwinia carotovora. Mol Microbiol. 1997;25:831–838. doi: 10.1111/j.1365-2958.1997.mmi501.x. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay M, Chakraborty D, Bhattacharyya S, Bhattacharya S. Regeneration of transformed plants from hairy roots of Plumbago indica. Plant Cell Tissue Organ Cult. 2010;102:109–114. doi: 10.1007/s11240-010-9702-z. [DOI] [Google Scholar]
- He-Ping S, Yong-Yue L, Tie-Shan S, Eric TPK. Induction of hairy roots and plant regeneration from the medicinal plant Pogostemon cablin. Plant Cell Tissue Organ Cult. 2011;107:251–260. doi: 10.1007/s11240-011-9976-9. [DOI] [Google Scholar]
- Inoguchi M, Ogawa S, Furukawa S, Kondo H. Production of an Allelopathic Polyacetylene in hairy root cultures of goldenrod (Solidago altissima L.) Biosci Biotechnol Biochem. 2003;67:863–868. doi: 10.1271/bbb.67.863. [DOI] [PubMed] [Google Scholar]
- Jahaniani F, Ebrahimi SA, Rahbar RN, Mahmoudian MX. Anthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochem. 2005;66:1581–1592. doi: 10.1016/j.phytochem.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Maknight TD, Lillis MT, Simpon RB. Segregation of genes transferred to one plant cell from two separated Agrobacterium strains. Plant Mol Biol. 1987;8:439–445. doi: 10.1007/BF00017989. [DOI] [PubMed] [Google Scholar]
- Mano Y, Matsuhashi M. A novel life cycle arising from leaf segments in plants regenerated from horseradish hairy roots. Plant Cell Rep. 1995;14:370–374. doi: 10.1007/BF00238599. [DOI] [PubMed] [Google Scholar]
- Montoro P, Teinseree N, Rattana W, Kongsawadworakul P, Michaux- Ferriere N. Effect of exogenous calcium on Agrobacterium tumefaciens-mediated gene transfer in Hevea brasiliensis (rubber tree) friable calli. Plant Cell Rep. 2000;19:851–855. doi: 10.1007/s002990000208. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog FA. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Porter RR. Host range and implication of plant infection by Agrobacterium rhizogenes. Crit Rev Plant Sci. 1991;10:387–421. doi: 10.1080/07352689109382318. [DOI] [Google Scholar]
- Rechinger KH (1982) Flora Iranica. AkademischeDruck-u.Verlagsanstalt, Graz. Vol 150
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Razavi K. Enhanced morphinan alkaloid production in hairy root cultures of Papaver bracteatum by over-expression of salutaridinol 7- o - acetyltransferase gene via Agrobacterium rhizogenes mediated transformation. World J Microbiol Biotechnol. 2013a doi: 10.1007/s11274-013-1377-2. [DOI] [PubMed] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Razavi K, Ntui VO. A reliable and efficient protocol for inducing hairy roots in Papaver bracteatum. Plant Cell Tissue Organ Cult. 2013;113:1–9. doi: 10.1007/s11240-012-0246-2. [DOI] [Google Scholar]
- Sharafi A, Hashemi Sohi H, Mousavi A, Azadi P, Hosseini Khalifani B, Razavi K. Metabolic engineering of morphinan alkaloids by over expression of codeinone reductase in transgenic hairy root of Papaver bracteatum. Biotechnol Lett. 2013;35:445–453. doi: 10.1007/s10529-012-1080-7. [DOI] [PubMed] [Google Scholar]
- Sobhani AM, Ebrahimi SA, Mahmoudian M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J Pharm Pharm Sci. 2002;5:19–23. [PubMed] [Google Scholar]
- Subotic A, Jevremovic S, Grubisic D, Jankovic T. Spontaneous plant regeneration and production of secondary metabolites from hairy root culture of Centaurium erythraea Rafn. Methods Mol Biol. 2009;547:205–215. doi: 10.1007/978-1-60327-287-2_17. [DOI] [PubMed] [Google Scholar]
- Sudha CG, Sherina TV, Anand VP, Reji JV, Padmesh P, Sonia EV. Agrobacterium rhizogenes mediated transformation of the medicinal plant Decalepis arayalpathra and production of 2-hydroxy-4-methoxy benzaldehyde. Plant Cell Tissue Organ Cult. 2012 [Google Scholar]
- Veena V, Taylor GG. Agrobacterium rhizogenes: recent developments and promising applications. In Vitro Cell Dev Biol. 2007;433:384–403. [Google Scholar]
- Winans SC. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kong J, Wang Y, Han Z, Xu X. Agrobacterium rhizogenes-mediated transformation and hairy root regeneration of Malus baccata (L.) Borkh. Acta Horticult Sinica. 2008;35:959–966. [Google Scholar]
- Wu J, Wang Y, Zhang LX, Zhang XZ, Kong J, Lu J, Han ZH. High-efficiency regeneration of Agrobacterium rhizogenes-induced hairy root in apple rootstock Malus baccata (L.) Borkh. Plant Cell Tissue Organ Cult. 2012;111:183–189. doi: 10.1007/s11240-012-0182-1. [DOI] [Google Scholar]