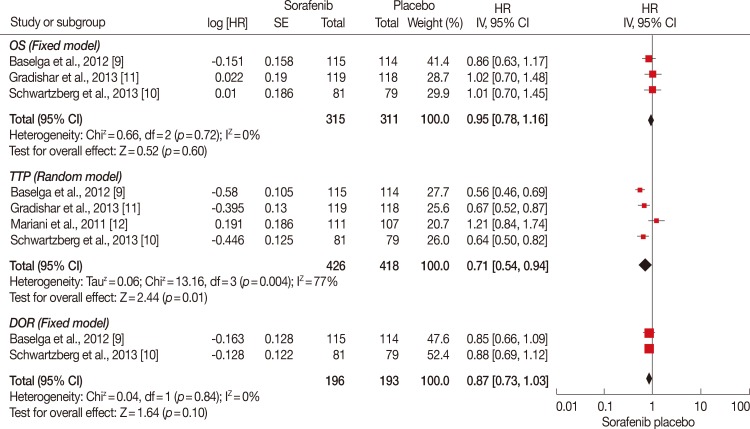
Figure 3.
Overall survival (OS), time to progression (TTP), and duration of response (DOR) analysis of sorafenib for human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer compared with placebo. TTP was significantly longer in sorafenib plus chemotherapy group (hazard ratio [HR], 0.71; 95% confidence interval [CI], 0.54-0.94; p=0.01). While OS and DOR were of no significance between the groups (HR, 0.95, 95% CI, 0.78-1.16, p=0.60; HR, 0.87, 95% CI, 0.73-1.03, p=0.10).
IV=inverse variance; SE=Standard Error.