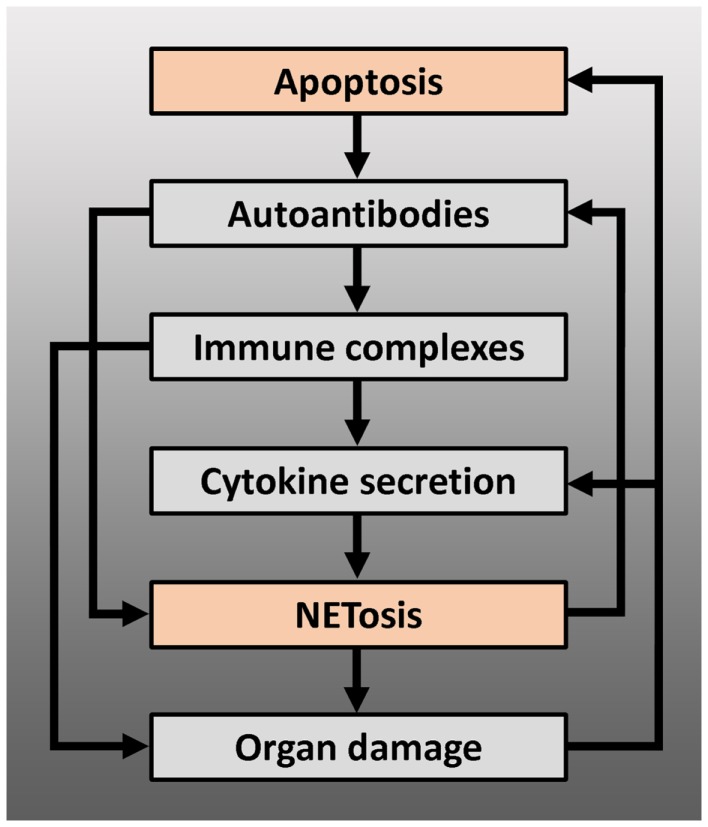
Figure 2.
Positive feedback loops arising from the interaction between apoptosis and NETosis, leading to chronification and/or exacerbation of the disease – modified autoantigens, derived from apoptotic cells, may be presented by antigen-presenting cells to autoreactive T cells, which can lead to production of autoantibodies by B cells, including anti-dsDNA or anti-RNP antibodies. These autoantibodies can induce NETosis or form immune complexes with their antigen. Immune complexes deposit on basal membranes, and incite a local inflammation (organ damage), or stimulate plasmacytoid dendritic cells to produce IFN-α and other pro-inflammatory cytokines. Pro-inflammatory cytokines such as IL-1β, TNF-α, or IFN-α induce NETosis or prime neutrophils for NETosis: NETs may serve as B cell autoantigens and lead to further autoantibody production or directly cause organ damage. Proteins from neutrophil granules, present in NETs, have shown to be highly toxic to glomerular structures and endothelium. Endothelial or glomerular damage causes further production of pro-inflammatory cytokines and leads to a new load of apoptotic cells.