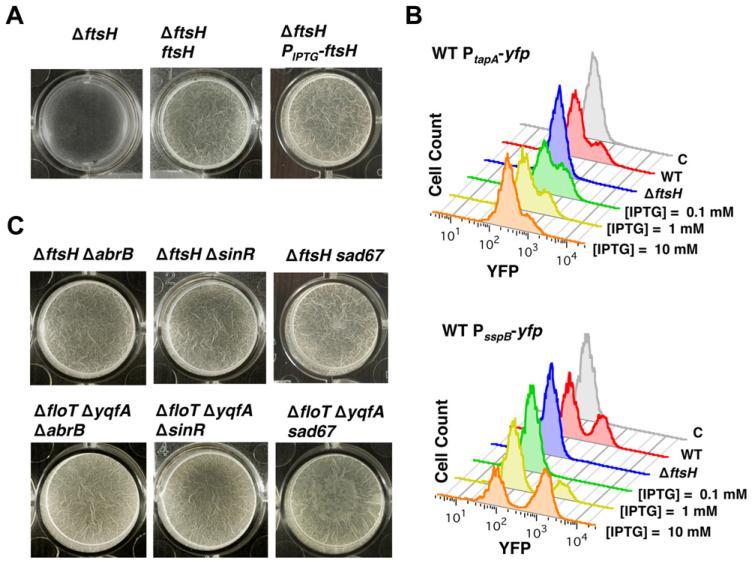
Fig. 6. Influence of FtsH on biofilm formation.
A. Pellicle formation of the ΔftsH mutant (DL1308, left panel), the ΔftsH mutant complemented with ftsH controlled by its native promoter (DL1433, middle panel) and ΔftsH mutant complemented with an IPTG-inducible promoter (DL1361, right panel). IPTG was added to a final concentration of 1 mM. Pictures show a top view of the pellicles formed on the surface of MSgg cultures incubated in 24-well plates at 30°C for 24 h.
B. Flow cytometry analysis of the same strains as in A harbouring PtapA-yfp reporter to monitor the differentiation of the subpopulation of matrix producers. Control strain harbouring no reporter fusion (grey profile). Wild-type (WT) profile shows a subpopulation of cells with high relative fluorescence, seen as the shoulder to the right of the main peaks (red profile) (DL382). The FtsH-defective mutant does not show the differentiation of this subpopulation (blue profile) (DL1404). Expression of ftsH under the control of an IPTG-inducible promoter led to a gradual expression of FtsH in the FtsH-defective mutant, which in turn caused a differentiation of matrix producers (different concentrations of IPTG are shown) (DL1461). Flow cytometry profiles of the reporter PsspB-yfp to detect sporulating cells. WT profile shows a subpopulation of cells with high relative fluorescence (red profile) (DL1089). The FtsH-defective mutant does not show the differentiation of this subpopulation (blue profile) (1349). Expression of ftsH under the control of an IPTG-inducible promoter led to a gradual expression of FtsH in the FtsH-defective mutant, which in turn caused a differentiation of sporulating cells (different concentrations of IPTG are shown) (DL1364).
C. Pellicle formation assay of the indicated strains of B. subtilis when incubated in MSgg at 30°C for 24 h. The sad67 variant was expressed under the control of an IPTG-inducible promoter with 1 μM IPTG.