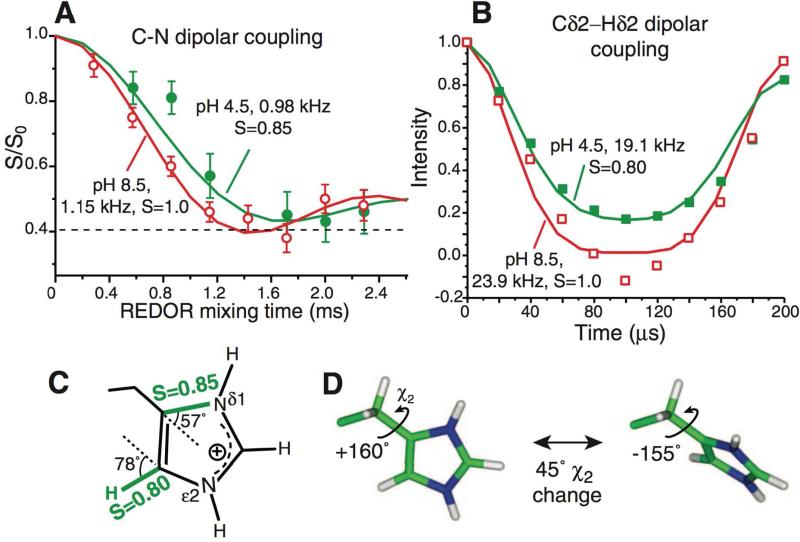
Figure 7.
Motions in influenza M2 proton channels. Histidine sidechains reorient at low pH but remain static at high pH at physiological temperature. A) 303 K 13C-15N dipolar couplings. At pH 8.5, a 1:1 combination of Cγ-Nδ1 and Cε1-Nδ1 couplings reaches the rigid limit. At pH 4.5, the dominant Cγ-Nδ1 coupling is motionally averaged. B) Cδ2-Hδ2 coupling at 308K is motionally averaged at pH 4.5 but in the rigid limit at pH 8.5. C) Measured order parameters at pH 4.5. D) Two-site jump of imidazolium at low pH. A 45° reorientation around the Cβ-Cγ bond fits the observed order parameters. Reprinted with permission from F. Hu, W. Luo, M. Hong, Science 2010, 330. 505-508, DOI: 10.1126/science.1191714. Copyright 2010 The American Association for the Advancement of Science (AAAS).