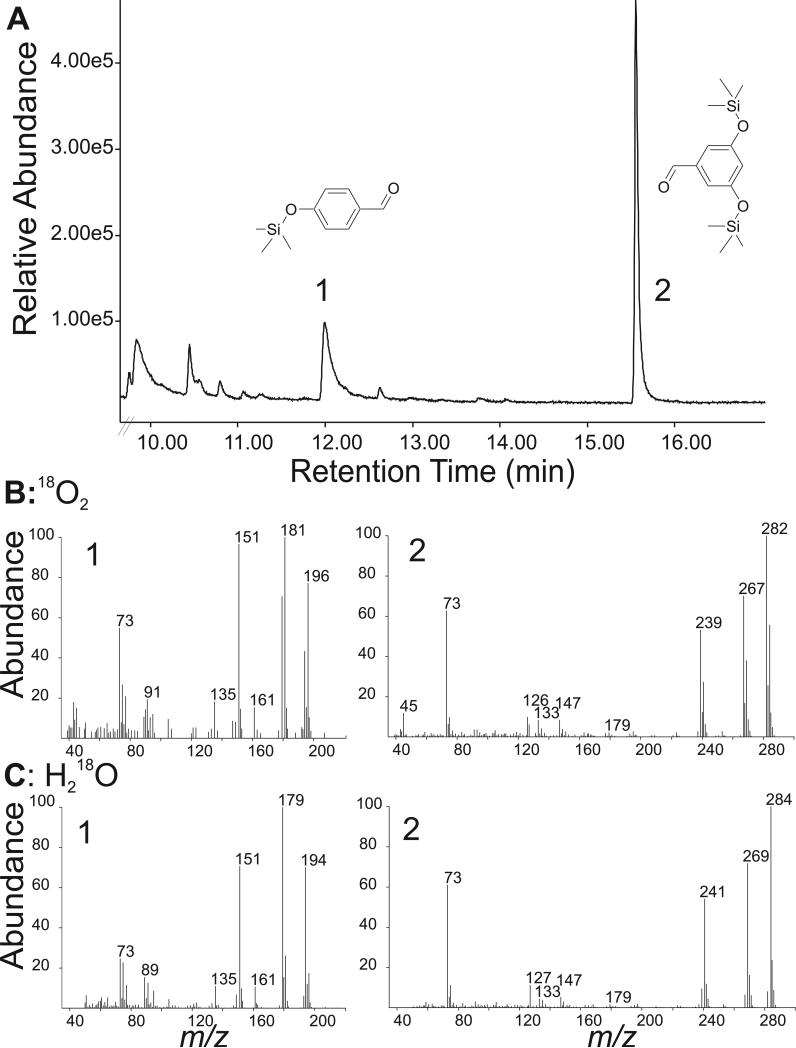
Figure 4.
GC-MS analysis of oxygen labeled cleavage products of resveratrol synthesized by NOV2. A) Silylated esters of cleavage products 4-hydroxybenzaldehyde (1) and 3,5-dihydroxybenzaldehyde (2). B) Incorporation of molecular oxygen into 4-hydroxybenzaldehyde. Mass spectra of 4-hydroxybenzaldehyde (left spectrum) and 3,5-dihydroxybenzaldehyde (right spectrum) in 18O2-atmosphere, showing labeled 4-hydroxybenzaldehyde (m/z 196) and unlabeled 3,5-dihydroxybenzaldehyde (m/z 282). C) Mass spectral data from incorporation of oxygen from water H218O. Incorporation of label into 3,5-dihydroxybenzaldehyde (m/z 284), but 4-hydroxybenzaldehyde remains unlabeled (m/z 194). Note that the main fragments in panels 1B and 1C (m/z 181 and m/z 179) result from the loss of a [–CH3] group from 4-hydroxybenzaldehyde.