Abstract
Background:
Helichrysum oligocephalum DC. from Asteraceae family is an endemic plant growing wild in Iran. This study was carried out to investigate the effect of H. oligocephalum hydroalcoholic extract (HOHE) on ulcerative colitis (UC) induced by acetic acid (AA) in rats.
Materials and Methods:
Rats were grouped (n = 6) and fasted for 24 h before colitis induction. Treatments were started 2 h before the induction of colitis and continued for two consecutive days with different doses of HOHE (100, 200, and 400 mg/kg) orally (p.o.) and intraperitoneally (i.p.). The colon tissue was removed and tissue damages were scored after macroscopic and histopathologic assessments.
Results:
Among the examined doses of HOHE, 100 mg/kg was the most effective dose that reduced the extent of UC lesions and resulted in significant alleviation. Weight/length ratio as an index of tissue inflammation and extravasation was also diminished in the treatment group administered HOHE at a dose of 100 mg/kg, and the results showed correlation with macroscopic and histopathologic evaluations. These data suggest that HOHE (100 mg/kg) administered either p.o. or i.p. was effective in diminishing inflammation and ulcer indices in this murine model of acute colitis in a non–dose-related manner.
Conclusions:
H. oligocephalum could be considered as a suitable anticolitis alternative; however, further studies are needed to support this hypothesis for clinical setting.
Keywords: Helichrysum oligocephalum, inflammation, rats, ulcerative colitis
INTRODUCTION
Inflammatory bowel diseases (IBD) are immune-mediated chronic or relapsing disorders of the gastrointestinal (GI) tract and consist mainly of Crohn's disease (CD) and ulcerative colitis (UC).[1]
The etiology of UC is still unknown; however, the most accepted hypothesis currently implicates a combination of one or more of the following factors:[2] increased free radical production and decreased antioxidant capacity, immune dysregulation caused by genetic or environmental factors, abnormal GI tract luminal factors such as the GI tract flora, and defects in the GI mucosal barrier that allow luminal factors to penetrate into the mucosa.[3,4,5]
Furthermore, infiltration of neutrophils, macrophages, lymphocytes and mast cells, ultimately giving rise to mucosal disruption and ulceration.[6]
Because the cause of IBD has not been fully established, current medical therapy is facilitative and supportive rather than curative.[7]
Therapeutic options include mesalamine and other 5-aminosalicylates (5-ASA), antibiotics (including rifaximin and tinidazole), steroids (including topical formulations and budesonide), immunomodulators [including azathioprine, 6-mercaptopurine, methotrexate (MTX), and cyclosporine], and monoclonal antibodies (including infliximab, adalimumab, and natalizumab).[8]
Inadequacies in efficacy and safety, potentially serious complications and side effects, and the costs of medicines provide a strong impetus to seek new approaches to disease management such as alternative and traditional therapies which are vastly considered in the IBD patients for several years.[9,10,11]
Herbal medicines are generally used in such cases when drugs are to be used for chronic periods.[12] Drugs of herbal origin reduce the offensive factors and have proved to be safe, clinically effective, relatively less expensive, and globally competitive with chemical drugs, and are with better patient tolerance.[13,14]
The genus Helichrysum belongs to the Asteraceae family and consists of an estimated 600 species.[15]
Nineteen species of the genus Helichrysum are found in Iran, of which eight are endemic. The rate of endemism in the genus Helichrysum in Iran is ca. 42%, and Helichrysum oligocephalum DC. is an endemic plant growing wild in Iran.[16,17]
The plant is spread over the entire country, but is most abundant in the west of Iran. This plant is currently offered for treatment of GI disease in Isfahan medicinal herbs market as a fraud type of Artemisia absinthium whose anti-ulcerative action has been demonstrated previously.[18,19]
Eighty-two components have been detected in the essential oil of H. oligocephalum, and thymol and beta-caryophyllene are the most important ones. Moreover, polyphenolic and flavonoids found in the hydroalcoholic extract of H. oligocephalum (HOHE) possess high antioxidant capacity with potent anti-inflammatory activity.[20]
Due to the above-mentioned properties, H. oligocephalum would be expected to reduce injury and/or improve colitis tissue in models with induced ulcerative colitis. We investigated the effects of this plant on tissue inflammatory activities in an acetic acid (AA)-induced ulcerative colitis model in rats.
MATERIALS AND METHODS
Plant material and preparation of extract
H. oligocephalum was purchased from a local medicinal herbs market in Isfahan and authenticated by Department of Pharmacognosy, Isfahan School of Pharmacy and Pharmaceutical Sciences. For preparation of HOHE, dried and finely powdered aerial parts of the plant (100 g) were wetted by ethanol:water mixture (70:30) and percolation was undertaken using extra volume of solvent for 48 h for full extraction. The extract was then shaken, filtered, and evaporated in a rotary evaporator under reduced pressure till an extract of semi-solid and gelling nature of yield 11% (w/w) was obtained.[21]
Chemicals
Dexamethasone was purchased from Iran Hormone Pharmaceutical Co. (Tehran, Iran). Hexadecyltrimethylammonium bromide (HTAB), aprotinin A, bovine serum albumin, phenylmethylsulfonyl fluoride, benzethonium chloride, ethylenediaminetetraacetic acid (EDTA), and Tween-20 were all purchased from Sigma Chemical Company (St. Louis, MO, USA). Tumor necrosis factor-alpha (TNF-α) (ALPCO, Windham, USA) kit was used for measurement of TNF-α variables. All the organic solvents and AA used were of analytical grade and procured from Merck (Darmstadt, Germany).
Animals
Seventy adult male Wistar rats (180–220 g) were used for this work (supplied by the Animal House, School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences). The animals were maintained under standard and constant conditions of light, humidity, and temperature. All rats were maintained on standard pelleted rat chow and water ad libitum. All experiments were conducted according to the local ethics guidelines for research on animals and approved by the Research Committee of Isfahan University of Medical Sciences.
Animal groups
Animals were randomly assigned to sham, control, test, and reference groups of six rats in each as follows:
Sham group: Treated with vehicle (distilled water) orally (p.o.) (1 ml) and intraperitoneally (i.p.) (0.5 ml) without colitis induction (normal group)
Control group: Treated with vehicle p.o. (1 ml) and i.p. (0.5 ml), respectively, after induction of colitis
Extract groups: Treated with HOHE at doses of 100, 200, and 400 mg/kg (1 ml p.o. and 0.5 ml i.p.)
Reference group: Treated with dexamethasone (2 mg/kg, p.o. and 1 mg/kg, i.p.)
The doses were given once daily starting 2 h before the induction of colitis and continued for two consecutive days. The test plant samples were freshly prepared prior to administration.
Induction of experimental colitis in rats
The animals were fasted for 24 h with free access to water before induction of colitis. Induction of colitis was performed using a modification of the method described by Mascolo et al. (1995).[22] Colitis was stimulated in the rats by AA-induced colonic inflammation under light ether anesthesia, administering 2 ml of 4% (v/v) AA intrarectally with a soft 6-Fr pediatric catheter. The catheter was inserted into the anus up to a length of 8 cm, and then AA was administered.
On the third day after induction, all rats were sacrificed. The last 8 cm of the colon was excised, opened longitudinally, and rinsed with normal saline solution. Then, the distal colon was weighed and the mucosal lesions were scored macroscopically. Tissue samples were taken for macroscopic scoring, histopathologic examination, and biochemical studies.
Assessment of colitis
After washing the mucosa with saline (0.9%) solution, the macroscopic appearance of the colonic mucosa was scored by an independent observer according to a scale ranging from 0 to 3 as follows:
0 = No ulcer
1 = Inflammation, edema, thickness, and superficial ulcer
2 = Bleeding and deep ulcer
3 = Necrosis and/or perforation
After macroscopic evaluations, the tissues were cut into three equal parts. One part of the sample of colonic tissue was preserved in 10% formalin for histologic examination and the other two colonic samples were frozen on liquid nitrogen and stored immediately at -70°C until analysis.
Determination of myeloperoxidase (MPO) activity
MPO activity has been used as an index of leukocyte adhesion and accumulation in several tissues including the intestine. The principle of the method depends on the release of MPO enzyme in the homogenate of the colonic tissue used. MPO activity was determined by the method of Motavallian et al.[23]
The tissue was weighed and then each sample was finely chopped in homogenizing tube with 2 ml potassium phosphate buffer (pH 6.0) containing 0.5% HTAB. After sonication, the homogenates were kept in an ice bath for 3 × 10 s, the suspensions were centrifuged at 15,000 rpm for 15 min at 4°C, and then the supernatant was decanted for analysis. Assay mixture was placed in a 1-cm path length cuvette that contained 0.1 ml of colonic supernatant and 2.9 ml of freshly prepared 50 mM phosphate buffer (pH 6.0) containing 0.005% H2O2 and o-dianisidine dihydrochloride (0.167 mg/ml). The supernatant was added last, and the change in absorbance at 450 nm was followed for 5 min. MPO activity was expressed in units (U) per gram tissue weight of wet tissue.
Measurement of TNF-α
TNF-α was determined according to the method of Motavallian et al.[23] The tissue samples were weighed, snap frozen on liquid nitrogen, and stored at -70°C to be processed for TNF-α determination. Colon samples were homogenized in phosphate-buffered saline (PBS; pH = 7.4) containing 0.4 M NaCl, 0.05% Tween-20, 0.5% bovine serum albumin, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, aprotinin A 20 KI, and 10 mM EDTA. They were then centrifuged at 12,000 ×g for 30 min at 4°C, and then enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of TNF-α in the supernatants.
Statistical analysis
Data analysis was performed using the SPSS statistical package (version 20.0) and the results were reported as mean ± standard error of mean (SEM). The differences between groups were tested by one-way analysis of variance (ANOVA) with Tukey's post-hoc test. Non-parametric data were analyzed by Mann–Whitney U test. P-value <0.05 was considered significant.
RESULTS
Macroscopic results
In the control group, severe macroscopic damage and hemorrhagic or ulcerated mucosa in the colon were found after rectal administration, as assessed by the colonic damage score scale.
Dexamethasone significantly decreased ulcer severity (P < 0.01 for p.o. and P < 0.05 for i.p.) and weight/length ratio (P < 0.05), compared to control group [Table 1].
Table 1.
Effect of H. oligocephalum hydroalcoholic extract (HOHE) on macroscopic and pathologic parameters and indices of colitis in rats
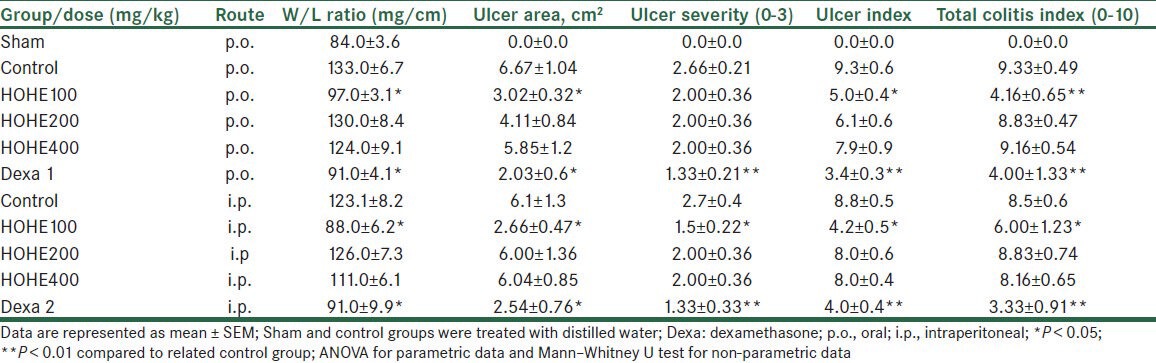
On the other hand, regardless of the mode of administration, H. oligocephalum (100 mg/kg) significantly reduced the ulcer severity in a non–dose-related manner (P < 0.05 in comparison to the control group) as shown in Table 1.
We found that ulcer area and ulcer index were decreased in dexamethasone and H. oligocephalum groups (100 mg/kg), compared to control group (P < 0.05) [Table 1].
Microscopic results
In the normal group, histopathologic assessment and microscopic images of the colon tissue revealed regular colonic mucosa with intact epithelium, crypts, and submucosa.
However, it was found that the AA-induced colitis group exhibited transmural necrosis, edema, and submucosal inflammatory cells’ infiltration and distortion of crypt architecture [Figure 1a].
Figure 1.
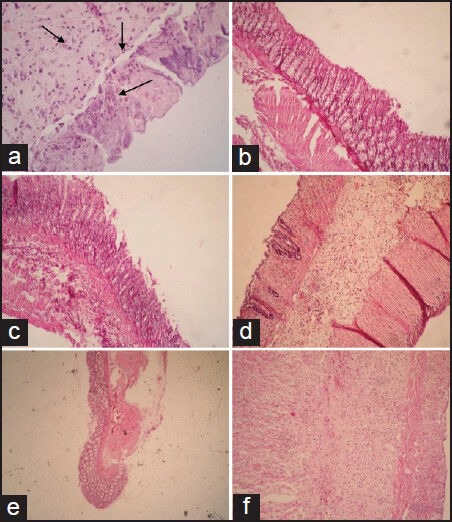
Microscopic presentation of acetic acid–induced colitis in rats. (a) Distortion of crypt architecture, loss of epithelial cells, ulceration, and neutrophil infiltration were observed in colitis rats. (b and c) Normal colonic tissue architecture was seen in dexamethasone (oral or parenteral) treated group. (d) H. oligocephalum (100 mg/kg) treated rats showed mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates. (e and f) H. oligocephalum treated rats (200 and 400 mg/kg) showed destruction of mucosal architecture and infiltration of neutrophils
Rats treated with dexamethasone [Figure 1b] or HOHE (100 mg/kg) [Figure 1c] significantly diminished neutrophil infiltration, submucosal edema, and the extent or severity of tissue damage, as reflected in total colitis index parameter [Table 1].
MPO activity
Colonic levels of MPO in different groups were assessed and compared with the level in control group. Colonic MPO activity in the control group was significantly higher than that in the normal group (P < 0.001), indicating increased leukocyte infiltration.
Treatment with either dexamethasone or HOHE (100 mg/kg) had similar effect on MPO activity in such a manner that both of them were effective in reducing the MPO level significantly as compared to that of the control group (P < 0.1) [Figure 2].
Figure 2.
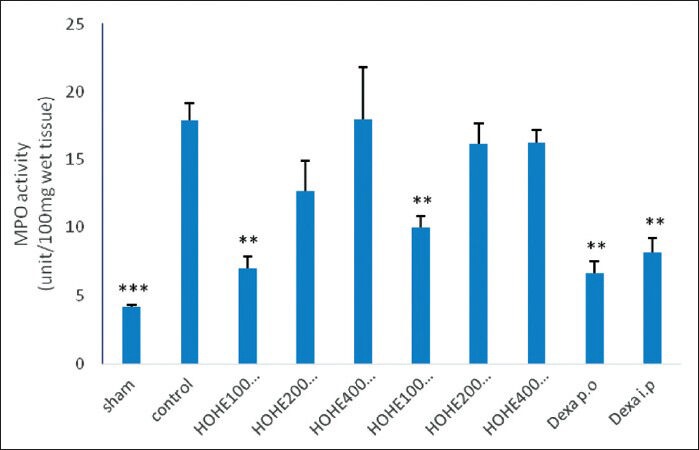
Effects of different doses of H. oligocephalum on colonic myeloperoxidase (MPO) levels in acetic acid–induced colitis in rats [HOHE, H. oligocephalum hydroalcholic extract (100, 200, 400 mg/kg); Dexa, dexamethasone (1 and 2 mg/kg); p.o., oral; i.p., intraperitoneal]
TNF-α levels
We found that TNF-α activity was significantly enhanced in the inflamed colon of control rats in comparison with that of sham group rats (P < 0.001).
Administration of HOHE (100 mg/kg), on the other hand, resulted in a significant reduction in colonic TNF-α level.
Greater doses of HOHE (200 and 400 mg/kg) did not show significant changes in macroscopic, histopathology, MPO activity, and TNF-α levels, as compared to those of control group [Table 1, Figures 1–3].
Figure 3.
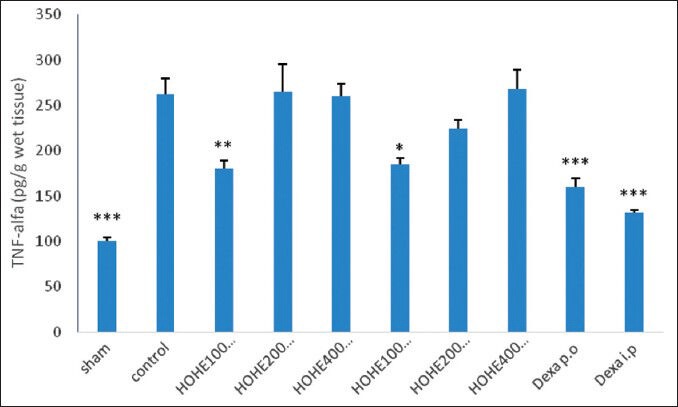
Effects of different doses of H. oligocephalum on colonic TNF-α levels in acetic acid–induced colitis in rats [TNF-alfa, tumor necrosis factor-alpha; HOHE, H. oligocephalum hydroalcholic extract (100, 200, 400 mg/ kg); Dexa, dexamethasone (1 and 2 mg/kg); p.o., oral; i.p., intraperitoneal]. Values are expressed as mean ± SEM of six rats per group
DISCUSSION
The present investigation demonstrated that intrarectal administration of 4% AA caused extensive macroscopic damage and formed hemorrhagic and ulcerated colonic mucosa.
Additionally, the present study showed a significant increase in MPO activity and in the level of some proinflammatory cytokines such as TNF-α in the control group.
The results obtained in the reference (dexamethasone) group, which showed an effective protection considering the macroscopic and microscopic outputs, in comparison to the control group indicated the consistency, effectiveness, and suitability of the method.
The effects of different traditional medicines have been studied on AA-induced colitis, including Matricaria aurea,[24] Zingiber officinale,[25] Ginkgo biloba,[26] Boswellia serrata,[27] and Calendula officinalis.[28]
The present study is the first to demonstrate the effect of H. oligocephalum on UC. Regarding the macroscopic (ulcer index) and histologic (total colitis index) results, it was evident that treating rats with HOHE (100 mg/kg) reduced the mucosal damage and subsequently attenuated MPO activity in colonic tissues.
Moreover, our study established that H. oligocephalum decreased the production of TNF-α in the colonic tissue. The most effective dose was 100 mg/kg, which shows that the active ingredients are potent. Higher doses (200, 400 mg/kg) administered either p.o. or i.p. had no significant therapeutic effect on inflammation and ulcerative indices in rat colons, suggesting that there is no relationship between dose and efficacy. It is notable that these higher doses of HOHE did not aggravate the colitis.
It could be attributed to some active components of extract which probably oppose with the above-mentioned therapeutic actions, and became more accentuated by using higher doses of HOHE. This is in accordance with the results reported by Bairy et al. They revealed that Amaranthus spinosus Linn. aqueous extract possesses diuretic property which is observed predominantly at 500 mg/kg dose and there is no dose–response relationship.[29]
Beta-thujone, one of the active ingredients of HOHE, has been shown to be toxic in greater amounts in rats.[30]
Preliminary phytochemical analysis of the studied extract revealed that flavonoides, saponins, and tannins are the main constituents. Thymol, on the other hand, is found in the greatest amount in the essential oil of H. oligocephalum, for which antioxidant,[31] anti-inflammatory,[32] and local anesthetic and analgesic[33] activities have been demonstrated, which can inhibit edema, inflammation, neutrophil migration, and TNF-α expression.[32,34]
Flavonoids have been reported to act in the GI tract, and have antispasmodic,[35] anti-secretory, antidiarrheal,[36] antiulcer, and antioxidant properties.[37]
The role of oxidative stress has been confirmed in IBD. These species are cytotoxic agents, causing cellular dysfunction and damage.[38]
Flavonoids react with the free radicals to form more stable radicals with lower toxicity. Besides, they can chelate Fe2+, which results in inhibiting the effects of free radicals.
Flavonoids can also inhibit the secretion of inflammatory mediators such as nitric oxide, interferon (INF)-γ, interleukin (IL)-12, and TNF-α, of which the latter has a principal role in IBD pathology.[39,40]
Saponins, which have anti-inflammatory, antioxidant, and anti-cancer effects, are the other ingredients found in significant amounts in HOHE.[41]
Tannins can protect intestinal mucosal layers by precipitating their microproteins, and also protect the layers against chemical injuries and proteolytic enzymes.[42]
CONCLUSION
In conclusion, this study suggests that H. oligocephalum possesses anti-inflammatory activity and improves ulcerative colitis at low doses and that it may be a promising therapeutic option for ulcerative colitis. More studies are strongly recommended to establish the mechanisms involved and the active constituents that are responsible for its beneficial pharmacologic actions.
Footnotes
Source of Support: This work was financially supported by Council of Research, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: The authors declare that have no conflict of interest.
REFERENCES
- 1.Klein A, Eliakim R. Non-steroidal anti-Inflammatory drugs and inflammatory bowel disease. Pharmaceuticals. 2010;3:1084–92. doi: 10.3390/ph3041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res. 2006;53:324–30. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 4.Kucharzik T, Maaser C, Lugering A, Kagnoff M, Mayer L, Targan S, et al. Recent understanding of IBD pathogenesis: Implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–83. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 5.Scaldaferri F, Fiocchi C. Inflammatory bowel disease: Progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171–8. doi: 10.1111/j.1751-2980.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiocchi C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 7.Kirsner JB. Inflammatory bowel disease. Part II: Clinical and therapeutic aspects. Dis Mon. 1991;37:669–746. doi: 10.1016/s0011-5029(05)80013-6. [DOI] [PubMed] [Google Scholar]
- 8.Poppers DM, Scherl EJ. Prophylaxis against pneumocystis pneumonia in patients with inflammatory bowel disease: Toward a standard of care. Inflamm Bowel Dis. 2008;14:106–13. doi: 10.1002/ibd.20261. [DOI] [PubMed] [Google Scholar]
- 9.Summers RW. Novel and future medical management of inflammatory bowel disease. Surg Clin N Am. 2007;87:727–41. doi: 10.1016/j.suc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Hay JW, Hay AR. Inflammatory bowel disease: Costs-of-illness. J Clin Gastroenterol. 1992;14:309–17. doi: 10.1097/00004836-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Bodger K. Cost of illness of Crohns disease. Pharmaeconomics. 2002;20:639–52. doi: 10.2165/00019053-200220100-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sairam K, Rao CV, Goel RK. Effect of Centella asiatica Linn on physical and chemical factors induced gastric ulceration and secretion. Indian J Exp Biol. 2001;39:137–42. [PubMed] [Google Scholar]
- 13.Goel RK, Sairam K. Antiulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasna, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol. 2002;34:100–10. [Google Scholar]
- 14.Sumbul S, Ahmad MA, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in- vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21–8. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozaffarian VA. Tehran: Farhang Moaser Publications; 1996. Dictionary of Iranian plant names; p. 488. [Google Scholar]
- 17.Hedge IC, Akhani H, Kothe-Heinrich G, Podlech D, Rilke S, Rechinger KH. Verlagsanstalt: Akademische Druck-u; 1997. Flora Iranica. [Google Scholar]
- 18.Gonzalez-Colomaa A, Bailena M, Diazb C, Fragab B, Martinez-Diazc R, Zunigad G, et al. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind Crops Prod. 2012;37:401–7. [Google Scholar]
- 19.Vahid Khori V, Nayebpour M. Effect of Artemisia absinthium on electrophysiological properties of isolated heart of rats. Physiol Pharmacol. 2007;10:303–12. [Google Scholar]
- 20.Sajjadi SE, Jafari A, Naderian M. Chemical composition of the essential oil of Helichrysum oligocephalum. Chem Nat Comp. 2009;45:269–71. [Google Scholar]
- 21.Ghassemi-Dehkordi N. 1st ed. Vol. 1. Tehran: Iranian Health Ministry Publications; 2002. Iranian herbal pharmacopoeia; pp. 387–96. [Google Scholar]
- 22.Mascolo N, Izzo A, Maiello A, Dicalo G, Coposso F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J Pharmacol Exp Ther. 1995;272:469–75. [PubMed] [Google Scholar]
- 23.Motavallian A, Minaiyan M, Rabbani M, Mahzuni P. Anti-inflammatory effect of ondansetron through 5-ht3 receptors on TNBS-induced colitis in rat. EXCLI J. 2012;11:30–44. [PMC free article] [PubMed] [Google Scholar]
- 24.Minaiyan M, Ghassemi-Dehkordi N, Mahzouni P, Ansari-Roknabady M. Effect of Matricaria aurea (Loefl.) Shultz-Bip. hydroalcoholic extract on acetic acid- induced acute colitis in rats. Iranian J Basic Med Sci. 2011;14:67–74. [Google Scholar]
- 25.Minaiyan M, Ghannadi A, Mahzouni P, Nabi-Meibodi M. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:15–22. [Google Scholar]
- 26.Mustafa A, El-Medany A, Hagar HH, El-Medany G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol Res. 2006;53:324–30. doi: 10.1016/j.phrs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann RM, Morgan Martins MI, Tieppo J, Fillmann HS, Marroni NP. Effect of Boswellia serrata on antioxidant status in an experimental model of colitis rats induced by acetic acid. Dig Dis Sci. 2012;57:2038–44. doi: 10.1007/s10620-012-2134-3. [DOI] [PubMed] [Google Scholar]
- 28.Mehrabani D, Ziaei M, Hosseini SV, Ghahramani L, Bananzadeh AM, Ashraf MJ. The effect of Calendula officinalis in therapy of acetic acid induced ulcerative colitis in dog as an animal model. Iran Red Crescent Med J. 2011;13:884–90. [PMC free article] [PubMed] [Google Scholar]
- 29.Amuthan A, Chogtu B, Bairy KL, Sudhakar, Prakash M. Evaluation of diuretic activity of Amaranthus spinosus Linn. aqueous extract in Wistar rats. J Ethnopharmacol. 2012;140:424–7. doi: 10.1016/j.jep.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 30.Patocka J, Pluca B. Pharmacology and toxicology of absinthe. J Appl Biomed. 2003;1:199–205. [Google Scholar]
- 31.Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, et al. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403:136–8. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Chagas-Paula DA, Oliveira RB, Da-Silva VC, Gobbo-Neto L, Gasparoto TH, Campanelli AP, et al. Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J Ethnopharmacol. 2011;136:355–62. doi: 10.1016/j.jep.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 33.Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol. 2002;19:571–9. doi: 10.1017/s0265021502000923. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan PS, Satti NK, Sharma VK, Dutt P, Suri KA, Bani S. Amelioration of inflammatory responses by chlorogenic acid via suppression of pro-inflammatory mediators. J Appl Pharm Sci. 2011;1:67–75. [Google Scholar]
- 35.Lima JT, Almeida JR, Barbosa-Filho JM, Assis TS, Silva MS, Dacunha EV, et al. Spasmolytic action of diplotropin, a furanoflavan from Diplotropis ferruginea Benth, involves calcium blockade in ginea-pig ileum. Z Naturforsch B. 2005;60:1–8. [Google Scholar]
- 36.Di Carlo G, Autore G, Izzo AA, Maiolino P, Mascolo N, Viola P, et al. Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure-activity relationships. J Pharm Pharmacol. 1993;45:1054–9. doi: 10.1111/j.2042-7158.1993.tb07180.x. [DOI] [PubMed] [Google Scholar]
- 37.La Casa C, Villegas I, Alarcon De La Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric Lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 38.Holma R, Salmenpera P, Riutta A, Virtanen I, Korpela R, Vapaatalo H. Acute effects of the cys-leukotriene-1 receptor antagonist, monteleukast, on experimental colitis in rats. Eur J Pharmacol. 2001;429:309–18. doi: 10.1016/s0014-2999(01)01330-9. [DOI] [PubMed] [Google Scholar]
- 39.Nijveldt RJ, Van-Nood E, Van-Hoorn DE, Boelens PG, Van-Norren K, Van-Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 40.Rao YK, Fang SH, Tzeng YM. Inhibitory effects of the flavonoids isolated from Waltheria indica on the production of NO, TNF-alpha and IL-12 in activated macrophages. Biol Pharm Bull. 2005;28:912–5. doi: 10.1248/bpb.28.912. [DOI] [PubMed] [Google Scholar]
- 41.Amzal H, Alaoui K, Tok S, Errachidi A, Charof R, Cherrah Y, et al. Protective effect of saponins from Argania spinosa against free radical-induced oxidative haemolysis. Can J Physiol Pharmacol. 2007;85:918–27. doi: 10.1016/j.fitote.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Aguwa CN, Nwako SO. Preliminary studies of the root extracts of Nauclea latifolia smith for anti-ulcer properties. Niger J Pharm Sci. 1988;4:16–23. [Google Scholar]


