Abstract
Inositol 1,4,5-trisphosphate [Ins(1,4,5)P3 1] mobilizes intracellular Ca2+ through the Ins(1,4,5)P3 receptor [InsP3R]. Although some progress has been made in the design of synthetic InsP3R partial agonists and antagonists, there are still few examples of useful small molecule competitive antagonists. A “multivalent” approach is explored and new dimeric polyphosphorylated aromatic derivatives were designed, synthesized and biologically evaluated. The established weak InsP3R ligand benzene 1,2,4-trisphosphate [Bz(1,2,4)P3 2] is dimerized through its 5-position in two different ways, first directly as the biphenyl derivative biphenyl 2,2′,4,4′,5,5′-hexakisphosphate, [BiPh(2,2′,4,4′,5,5′)P6 8] and with its regioisomeric biphenyl 3,3′,4,4′,5,5′-hexakisphosphate [BiPh(3,3′,4,4′,5,5′)P6 11]. Secondly, a linker motif is introduced in a flexible ethylene-bridged dimer (9) with its corresponding 1,2-bisphosphate dimer (10), both loosely analogous to the very weak antagonist 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA 7). In permeabilized L15 fibroblasts overexpressing type 1 InsP3R, BiPh(2,2′,4,4′,5,5′)P6 (8) inhibits Ins(1,4,5)P3-induced Ca2+ release in a apparently competitive fashion [IC50 187 nM] and the Bz(1,2,4)P3 dimer (9) is only slightly weaker [IC50 380 nM]. Compounds were also evaluated against type I Ins(1,4,5)P3 5-phosphatase. All compounds are resistant to dephosphorylation, with BiPh(2,2′,4,4′,5,5′)P6 (8), being the most effective inhibitor of any biphenyl derivative synthesized to date [IC50 480 nM] and the Bz(1,2,4)P3 ethylene dimer (9) weaker [IC50 3.55 μM]. BiPh(3,3′,4,4′,5,5′)P6 (11) also inhibits 5-phosphatase [IC50 730 nM] and exhibits unexpected Ca2+ releasing activity [EC50 800 nM]. Thus, relocation of only a single mirrored phenyl phosphate group in (11) from that of antagonist (8) does not markedly change enzyme inhibitory activity, but elicits a dramatic switch in Ca2+-releasing activity. Such new agents demonstrate the power of the multivalent approach and may be useful to investigate the chemical biology of signaling through InsP3R and as templates for further design.
Keywords: Ins(1,4,5)P3 receptor; antagonist; benzene polyphosphate; biphenyl polyphosphate; competitive inhibition
Introduction
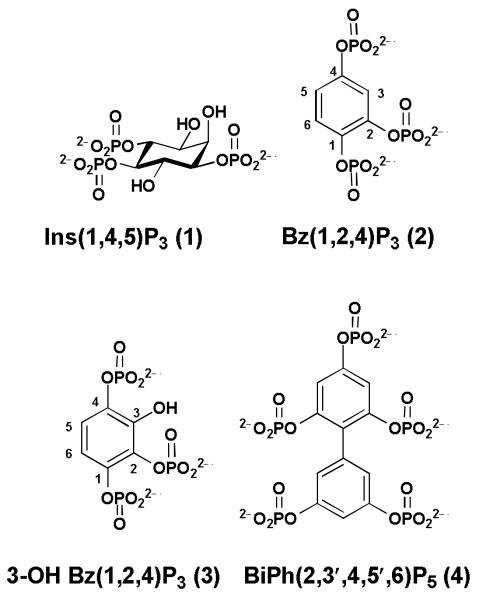
Ins(1,4,5)P3 receptors [InsP3Rs] are intracellular ligand-gated Ca2+ release channels present in the endoplasmic reticulum, which is the major storage site for Ca2+ in the cell. Ca2+ is released when Ins(1,4,5)P3 (1, Figure 1) binds to its receptor, eliciting many intracellular cellular responses including regulation of gene expression, cell division, synaptic transmission etc [Foskett, 2007]. In mammals three different isoforms of InsP3R are expressed with a high proportion of the type 1 receptor [InsP3R1] found in the brain [Foskett, 2007]. The InsP3R is made up of four subunits and the N-terminal region (residues 1-604) contains the Ins(1,4,5)P3 ligand binding (224-604) and suppressor domains (1-223). The crystal structure of the ligand binding domain of InsP3R1 (residues 224-604) was described [Bosanac, 2002] and more recently structures in the presence and absence of Ins(1,4,5)P3 [Lin, 2011] and for the full N-terminal domain (residues 1-604) [Seo, 2012] have been reported. There are, however, no crystal structures containing partial agonists or antagonists which may provide further clues to the activation mechanism of the InsP3R. Numerous groups have been engaged in synthesizing both natural and non-natural inositol polyphosphates and analogues since the discovery of Ins(1,4,5)P3 [Potter, 1995].
Figure 1. Myo-inositol 1,4,5-trisphosphate and some known aromatic polyphosphates.
After Ins(1,4,5)P3 releases Ca2+ from intracellular stores it diffuses away from the InsP3R and is hydrolysed by type I Ins(1,4,5)P3 5-phosphatase [Verjans,1994]. Type I 5-phosphatase enzyme is to a large extent membranous and ubiquitous [Storey, 1984, Erneux 1986], one of a family of ten Mg2+ dependent 5-phosphatase enzymes [Verjans, 1994] and is the only 5-phosphatase enzyme that hydrolyzes Ins(1,4,5)P3 to Ins(1,4)P2 [Erneux, 1989].
Heparin is a well-known InsP3 antagonist [Bultynck, 2003]. There are a number of small molecule Ins(1,4,5)P3 receptor antagonists currently used for biological studies, including 2-aminoethoxydiphenyl borate, xestospongins and caffeine, but none is competitive and specific for the InsP3R [Bootman, 2002, Bultynck, 2003] and results are often unreliable. myo-Inositol 1,3,4,5-tetrakisphosphate at high concentration is known to inhibit the InsP3R and several other phosphorylated derivatives can displace bound [3H]Ins(1,4,5)P3 at low to high micromolar concentration [Hermosura, 2000]. Inositol trisphosphorothioates, such as D-6-deoxy inositol 1,4,5-trisphosphorothioate [Safrany, 1993] and 1l-chiro-inositol 2,3,5-trisphosphorothioate [Safrany, 1993, Liu, 1994], myo-inositol 1,4,6-trisphosphorothioate and the related 1,3,6-trisphosphorothioate [Murphy, 2000] are competitive antagonists of the InsP3R in platelets, but are also very low efficacy partial agonists because they are still able to release a fraction of the Ca2+ store. Only one report of a small molecule full InsP3R competitive antagonist has been made, based upon the inositol core [Keddie, 2011], but the reported derivatives only very weakly inhibit the InsP3R at a millimolar level.
Aromatic polyphosphate derivatives have proved to be of potential in the phosphoinositide field. Benzene 1,2,4-trisphosphate [Bz(1,2,4)P3 (2)] (Figure 1) with phosphate groups arranged around a six-membered ring in a similar way to Ins(1,4,5)P3 was synthesized and evaluated against three proteins that bind Ins(1,4,5)P3 (1), the InsP3R, Ins(1,4,5)P3 type I 5-phosphatase and Ins(1,4,5)P3 3-kinase [Poitras, 1993]. Bz(1,2,4)P3 is resistant both to dephosphorylation by 5-phosphatase and phosphorylation by Ins(1,4,5)P3 3-kinase. Introduction of a 3-hydroxyl motif into Bz(1,2,4)P3 to give 3-hydroxybenzene 1,2,4-trisphosphate [3-OH-Bz(1,2,4)P3 (3)] however, transforms the parent compound into a substrate for Ins(1,4,5)P3 5-phosphatase [Mills, 2006]. More recently, several benzene polyphosphates, including Bz(1,2,4)P3 were also evaluated against Ins(1,4,5)P3 3-kinase but found to be ineffective at inhibiting the enzyme [Vandeput, 2007]. Interestingly, earlier data show that Bz(1,2,4)P3 weakly interacts with the InsP3R [Poitras, 1993], and competitively blocks [3H]Ins(1,4,5)P3 binding 10,000-fold more weakly than Ins(1,4,5)P3. Another study [Ward, 1995] showed that Bz(1,2,4)P3 inhibits in vitro phosphatidylinositol 3-kinase activity. More recently, our biphenyl derivative BiPh(2,3′,4,5′,6)P5 (4) (Figure 1) was found to be a moderately potent Ins(1,4,5)P3 receptor antagonist having an IC50 value in the low micromolar range [Vandeput, 2007]. Preliminary structure activity relationship (SAR) data indicate that the number and position of the phosphate groups might influence the recognition of benzene phosphate derivatives.
Our previous studies show that benzene polyphosphates are non-hydrolysable Ins(1,4,5)P3 5-phosphatase inhibitors [Mills, 2008] and biphenyl 2,3′,4,5′,6-pentakisphosphate [BiPh(2,3′,4,5′,6)P5 (4)] [Vandeput, 2007], the first aromatic polyphosphorylated ligand with activity to possess more than one ring, is one of the most potent Ins(1,4,5)P3 5-phosphatase inhibitors that also inhibits Ins(1,4,5)P3-induced Ca2+ release.
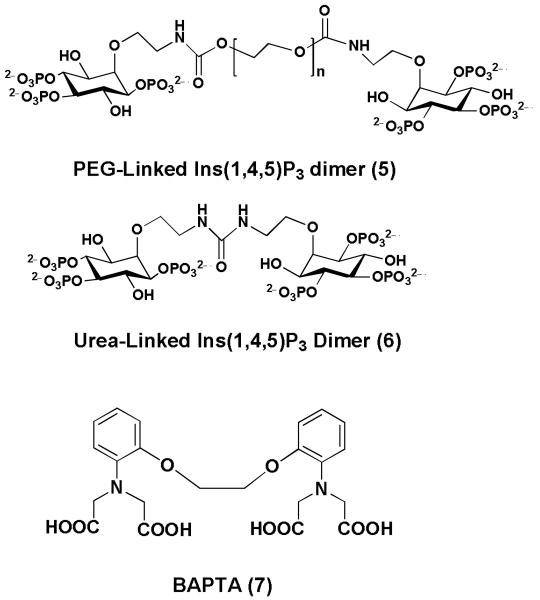
Multivalency is the use of two or more active groups to provide bioactivity that is more than additive. A new type of dimeric inositol polyphosphate derivative (5) (Figure 2) was designed when two molecules of Ins(1,4,5)P3 were synthetically linked via a polyethylene glycol spacer [Riley, 2002]. These dimers stimulate release of Ca2+ from permeabilized cells and are highly potent. A subsequent study [Riley, 2004] showed that an Ins(1,4,5)P3 dimer linked via the 2-hydroxyl group with a short N,N-diethylurea spacer (6) (Figure 2) has an EC50 value more than 12-fold lower than Ins(1,4,5)P3 and is the most potent in the series. Further modifications of these ligands led to the discovery of their partial agonist properties [Rossi, 2009]. The multivalent approach thus offers attractive design potential and, with the established high potency of the multivalent short Ins(1,4,5)P3 dimer (6), we therefore explored the possibility of further modification to the biphenyl core lead structure by both changing the number and regiochemistry of the decorating phosphates and bridging the two phosphorylated aromatic rings using a linker.
Figure 2. Structures of Ins(1,4,5)P3 dimers and BAPTA.
In the search for small molecule InsP3R antagonist leads, our attention also focused on the Ca2+ indicator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA 7, Figure 2), a weak competitive antagonist at the InsP3R [Richardson, 1993, Morris, 1999]. The carboxylic acid groups and nitrogen heteroatoms are required to chelate Ca2+, [Morris, 1999]. BAPTA competitively antagonizes Ins(1,4,5)P3-stimulated Ca2+ mobilization (KD = 1.8 mM). Although there is no speculation as to how molecules such as BAPTA are supposed to bind to the InsP3R, its non-chelated carboxylic acids might obviously bind weakly to the extensive network of positively charged amino acids in the Ins(1,4,5)P3 binding site. We hypothesised that such binding by BAPTA might be enhanced by replacing the carboxylic acids with other negatively charged groups.
We report here the synthesis of new dimeric benzene polyphosphates as both biphenyl derivatives and with short ethylene linkers akin to BAPTA (compounds 8-11, Figure 3). Thus, the weak ligand Bz(1,2,4)P3 (2) was dimerized through its 5-position in two different ways, first directly as a biphenyl derivative and secondly by introducing a BAPTA-type linker motif. These compounds thus both develop further the earlier biphenyl template antagonist lead [Vandeput, 2007] and also exemplify a new, more flexible, related class. Compounds were evaluated for activity using permeablized L15 fibroblasts overexpressing InsP3R1 and also on recombinantly expressed human type I Ins(1,4,5)P3 5-phosphatase.
Figure 3. Structures of dimeric aromatic polyphosphates.
Materials and methods
Chemistry
General
Chemicals were purchased from Acros, Sigma-Aldrich, and Alfa Aesar. Thin-layer chromatography (TLC) was performed on pre-coated plates (Merck TLC aluminium sheets silica 60 F254): products were visualized by dipping plates in an ethanolic solution of phosphomolybdic acid then heating at high temperature. Organic compounds were dried over MgSO4. Flash chromatography was carried out on Fisher Scientific Silica 60A (particle size 35-70 micron). All final compounds were evaluated by standard spectroscopic methods and purified by ion exchange chromatography performed on an LKB-Pharmacia Medium Pressure Ion Exchange Chromatograph using Q-Sepharose Fast Flow with gradients of triethylammonium bicarbonate (TEAB, 0→2.0 m) as eluent. Column fractions containing benzene polyphosphates were identified by U.V. spectroscopy at 254 nm and were quantified for total phosphate by a modification of the Briggs test [Lampe, 1994]. NMR spectra (proton frequency 270 or 400 MHz) were referenced against SiMe4, (HDO), [D6-(CD3)2CO], [D3-(CD3CN)] or [D6-(CD3)2SO], and 13C NMR was carried out at 100 MHz. The 31P NMR shifts (phosphorus frequency 162 MHz) were measured in ppm relative to external 85% phosphoric acid. Since the NMR spectra of all final compounds were recorded as their triethylammonium salts in D2O, the pH was ca. 5. Melting points (uncorrected) were determined using a Reichert-Jung Thermo Galen Kofler block or a Stanford Research Systems Optimelt automated melting point system. Microanalysis was carried out at the University of Bath. Mass spectra were recorded using electrospray (ES) with sodium formate as standard or Fast Atom Bombardment (FAB) where the standard was 3-nitrobenzyl alcohol (NOBA).
All final compounds were homogeneous, used as their triethylammonium salt after purification by ion exchange chromatography and were quantified by total phosphate assay [Lampe, 1994]. The latter procedure is more accurate than quantification by mass due to unknown amounts of residual water, inaccurate determination of the variable amount of triethylammonium counter ion and the inaccuracy in weighing out small quantities of material. All the final phosphorylated compounds are air stable and do not decompose when left in solution around pH 7, at least for a short time. 1.0 Micromole samples of compound in solution in an Eppendorf tube were carefully evaporated in a speedvac providing a pellet that was then used in biological studies.
2,2′,4,4′,5,5′-Hexamethoxybiphenyl (13)
MoCl5 (8.19 g, 30 mmol) was added to a solution of 1,2,4-trimethoxybenzene (12) (2.52 g, 15 mmol) (Rf = 0.24, CH2Cl2) in anhydrous CH2Cl2 (50 mL) at room temperature under Argon. The solution turned dark green and the heterogeneous mixture was stirred for 30 min under nitrogen. MeOH (50 mL) was then added to the reaction mixture and the solvents were evaporated off to give an olive green residue and a red colouration. The remaining solid was partitioned between CH2Cl2 and water (100 mL of each), and the red colour was present in the aqueous layer and the green colour in the organic layer. The organic layer was filtered through filter paper and the solvent was evaporated to give a pale green solid. The crude material was suspended in MeOH and the resulting white solid was filtered off and washed with ether (100 mL). Rf = 0.39, (ether), Rf = 0, (CH2Cl2). (The compound is only soluble in cold CH2Cl2, not EtOAc). The compound was recrystallized from MeOH to give the title compound (13) as a white crystalline solid (2.24 g, 89 %), (crystals were rod-like prisms) m.p. = 177-179 °C; Lit. 179 °C. 1H NMR (400 MHz, CDCl3) 3.74, 3.83, 3.92 (3 s, 18 H, 6 × ArOMe), 6.61, 6.81 (2 s, 4 H, ArH); 13C NMR (100 MHz, CDCl3) 56.06, 56.50, 56.85 (3 q, 6 × ArOMe), 98.35, 115.31 (2 d, ArH), 118.91 (s, Cq Ar-Ar), 142.86, 148.77, 151.22 (3 s, Cq, ArOMe); (HRMS, ESI+) m/z Calcd for C18H23O6 [M + H]+ 335.1489. found 335.1488.
2,2′,4,4′,5,5′-Hexahydroxybiphenyl (14)
2,2′,4,4′,5,5′-Hexamethoxybiphenyl (13) (1.003 g, 3.0 mmol) was partially dissolved in dry CH2Cl2 (10 mL) and the solution was cooled using a dry ice acetone mixture. A solution of BBr3 in CH2Cl2 (1.0 M, 25 mL) was added over 5 min to the cooled solution to give a pale green-yellow solution, which was then allowed to warm to ambient temperature over a period of 19 h. An aqueous solution of 1 M HCl (50 mL) was added to the cooled mixture (dry ice-acetone) which resulted in a white precipitate. Water (100 mL) was then added and the layers separated. The aqueous layer was extracted with ethyl acetate (4 × 100 mL), dried (MgSO4) and the solvent was evaporated to give a solid which had a violet colouration. The remaining solid was suspended in ether (40 mL) to dissolve any of the impurities and filtered to give the title compound as a violet (blue grey) coloured solid (14) (633 mg, 84%). The solid was not recrystallized but was pure enough for phosphorylation and pure by NMR. 1H NMR (400 MHz, d6-DMSO) 6.33, 6.50 (2 s, 4 H, ArH), 8.21, 8.51, 8.72 (3 s, 6 H, D2O exch, 6 × ArOH); 13C NMR (100 MHz, d6-DMSO) 104.28, 116.44 (2 d, 4 × CH, ArH), 117.78 (s, Cq, Ar-Ar), 138.165, 144.65, 146.09 (3 s, Cq, ArOH); (HRMS, ESI+) m/z Calcd for C12H11O6 [M + H]+ 251.0550. found 251.0549.
2,2′,4,4′,5,5′-Hexakis(diethoxyphosphoryloxy)biphenyl (15)
A mixture of diethyl chlorophosphite (1.33 mL, 7.8 mmol) and N,N-diisopropylethylamine (1.75 mL, 10.0 mmol) was stirred at room temperature in dry CH2Cl2 (10 mL) to give a yellow solution. 2,2′,4,4′,5,5′-Hexahydroxybiphenyl (14) (250 mg, 1 mmol) was added in small portions and the solid dissolved with the aid of ultrasound within 5-10 min with the solution remaining a yellow colour which was stirred for a further 30 min. The mixture was cooled using dry ice in acetone, then mCPBA (2.58 g, 15 mmol) in CH2Cl2 (25 mL) was added in one portion, the colour went a dark olive green and the solution was stirred for a further 30 min. Due to work up (sodium metabisulphite & NaHCO3 wash) also destroying the compound, the mixture was purified directly by flash chromatography using EtOAc then EtOAc-EtOH (5:1) to give compound (15) as a syrup, (580 mg, 54 %), Rf = 0.25 (EtOAc-EtOH, 5:1). 1H NMR (400 MHz, CDCl3) 1.19-1.22 (12 H, t, J = 7.0 Hz 2 × ArOP(O)(OCH2CH3)2), 1.34-1.38 (24 H, 2 t, J = 7.0 Hz, 4 × ArOP(O)(OCH2CH3)2), 3.91-4.01 (8 H, m, 2 × ArOP(O)(OCH2CH3)2), 4.18-4.30 (16 H, m, 4 × ArOP(O)(OCH2CH3)2), 7.33 (2 H, d, J = 0.8 Hz, CH, Ar), 7.53 (2 H, d, J = 0.8 Hz, CH, Ar); 13C NMR (100 MHz, CDCl3) 15.82, 15.88, 16.00, 16.07 (4 q, 6 × ArOP(O)(OCH2CH3)2), 64.76, 64.82, 64.85, 64.91, 64.97, 65.04 (6 t, 6 × ArOP(O)(OCH2CH3)2), 113.27, 123.90 (2 d, 4 × CH, Ar), 124.55, 124.63 (Cq, 2 × Ar-Ar), 138.11, 138.16, 141.69, 144.79, 144.85 (Cq, 6 × ArOP(O)(OCH2CH3)2); 31P NMR (162 MHz, CDCl3) −6.43 (2 × ArOP(O)(OCH2CH3)2), −6.70 (2 × ArOP(O)(OCH2CH3)2), −7.17 (2 × ArOP(O)(OCH2CH3)2). (HRMS, ESI+) m/z Calcd for C36H65O24P6 [M + H]+ 1067.2285. found 1067.2270, calcd for C36H64O24P6 C 40.53, H 6.05; found: C 40.3, H 5.73.
2,2′,4,4′,5,5′-Biphenylhexakisphosphate (8)
2,2′,4,4′,5,5′-Hexakis(diethoxyphosphoryloxy)biphenyl (15) (100 mg, 93.7 μmol), was dissolved in dry CH2Cl2 (5 mL). Bromotrimethylsilane (1.0 mL, 7.57) was added and the solution was stirred for 3 days, each day monitoring the disappearance of the ethyl groups from the compound. The solvents were evaporated and the remaining syrup was stirred in a mixed solvent of TEAB (1 mL) and water (2 mL) for 30 min. The title compound was purified over Q-Sepharose Fast Flow using a linear gradient of 0→2.0 M TEAB, eluting at 2.0 M buffer, and compound (8) was obtained as a glassy triethylammonium salt, (72.4 μmol, 77%). 1H NMR (400 MHz, D2O) 7.24, 7.28 (4 H, 2 s, ArH); 13C NMR (100 MHz, D2O) 113.25, 124.63 (2 d, 4 × CH, Ar), 123.90, 123.98 (Cq, 2 × Ar-Ar), 139.20 (Cq, t, J = 5.9 Hz, ArOPO32−), 143.43 (Cq, t, J = 5.9 Hz, ArOPO32−), 145.76 (Cq, d, J = 5.9 Hz, ArOPO32−); 31P NMR (162 MHz, D2O) −2.28, −2.50, −3.17 (3 s, 6 × ArOPO32−), (HRMS, ESI−) m/z Calcd for C12H15O24P6 [M − H]− 728.8384. found 728.8357.
2,4,5-Tribenzyloxyphenol (18)
mCPBA (2.9 g, 16.8 mmol) was added to a solution of 2,4,5-tribenzyloxybenzaldehyde (16) (4.245 g, 10 mmol) in dry CH2Cl2 (75 mL) and the mixture was stirred at room temperature for 18 h. The organic layer was washed with an aqueous solution of 10% sodium metabisulfite (2 × 100 mL) a saturated solution of sodium hydrogen carbonate (100 mL) and water (100 mL). The organic layer was filtered and the solvent was evaporated to give the crude formate ester derivative (17) (Rf = 0.50, CH2Cl2) which was purified by flash chromatography (CH2Cl2). The resulting solid was dissolved in a mixed solvent (CH2Cl2, 25 mL and MeOH, 25 mL) and 5 drops of concentrated hydrochloric acid were added. The reaction was stirred for 90 min and the solvents were evaporated to give the crude product. Purification of the title compound (18) was achieved using flash chromatography (CH2Cl2) to give the product as a solid, (2.73 g, 66%), (Rf = 0.40, CH2Cl2); m.p. 75-77 °C from ether-hexane. 1H NMR (400 MHz, CDCl3) 4.91, 5.00, 5.03 (6 H, 3 s, 3 × ArOCH2Ph), 5.30 (1 H, s, ArOH, D2O exch.), 6.60 (1 H, s, CH, Ar), 6.61 (1 H, s, CH, Ar), 7.24-7.40 (15 H, m, 3 × ArOCH2Ph); 13C NMR (100 MHz, CDCl3) 71.81, 71.97, 73.30 (3 t, ArOCH2Ph), 103.48, 105.08 (2 d, CH, Ar), 127.15, 127.45, 127.49, 127.50, 127.54, 128.05, 128.08, 128.13, 128.37 (d, ArOCH2Ph), 136.08, 136.92, 137.18, 138.96, 140.66, 141.31, 144.19 (Cq, ArOCH2Ph, ArOH, ArOBn); MS: (FAB)+ 91, 412.3; (HRMS, ESI+) m/z Calcd for C27H25O4 [M + H]+ 413.1747. found 413.1739, calcd for C27H24O4 C 78.62, H 5.86; found: C 78.8, H 5.88.
1,2-Bis(2,4,5-tris(benzyloxy)phenoxy)ethane (19)
A mixture of 2,4,5-tribenzyloxyphenol (18) (2.73 g, 6.61 mmol), ethylene glycol di-p-tosylate (1.22 g, 3.3 mmol) and K2CO3 (2.76 g, 20 mmol) was heated at 130 °C under N2 in dry DMF (50 mL) for 22 h. The dark coloured DMF was evaporated and the remaining solid was partitioned between water and CH2Cl2 (200 mL of each) and the organic solvent was evaporated to give the crude product Rf = 0.26 (CH2Cl2) and lower Rf than the starting material Rf = 0.40 (CH2Cl2) on same TLC plate. The remaining solid was subject to flash chromatography (CH2Cl2) to give compound (19) as a pure crystalline white solid. Yield = (1.483 g, 53%) (EtOAc-petroleum ether 40-60 °C), m.p. = 128-129 °C. 1H NMR (400 MHz, CDCl3) 4.21 (4 H, s, (OBn)3ArOCH2CH2OAr(OBn)3), 4.95, 4.99, 5.00 (12 H, 3 s, 6 × ArOCH2Ph), 6.61, 6.67 (4 H, 2 s, ArH), 7.25-7.38 (30 H, m, 6 × ArOCH2Ph); 13C NMR (100 MHz, CDCl3) 69.32 (t, (OBn)3ArOCH2CH2OAr(OBn)3), 72.49, 72.51, 72.60 (3 t, 6 × ArOCH2Ph), 106.89, 107.28 (2 d, 4 × ArH), 127.53, 127.60, 127.76, 127.79, 128.39, 128.40 (d, ArOCH2Ph), 137.25, 137.29, 143.53, 143.64, 143.71, 143.76 (Cq, ArOCH2Ph, ArOCH2Ph). (HRMS, ESI+) m/z Calcd for C56H50O8Na [M + Na]+ 873.3398. found 873.3406, calcd for C56H50O8 C 79.04, H 5.92; found: C 78.9, H 5.87.
5,5′-(Ethane-1,2-diylbis(oxy))dibenzene-1,2,4-triol (20)
1,2-Bis(2,4,5-tris(benzyloxy)-phenoxy)ethane (19) (1.254 g, 1.47 mmol) was dissolved in a mixed solvent of CH2Cl2 (50 mL) and MeOH (10 mL) and palladium on carbon (10%, 100 mg) was added. The air was expelled and the solution was stirred over an atmosphere of hydrogen for 18 h at room temperature. A further portion of MeOH (40 mL) was added and the mixture was hydrogenated for a further 4 h to ensure full deprotection. The colourless solution was filtered off and washed with more MeOH and the solvent was evaporated to yield a pale pink-grey-purple solid which was then washed with ether (439 mg, 1.415 mmol, 96%), compound (20) was not recrystallized. 1H NMR (400 MHz, d6-DMSO) 4.04 (s, 4 H, (OH)3ArOCH2CH2OAr(OH)3), 6.29, 6.41 (4 H, 2 s, ArH), 8.09 (4 H, s, D2O exch. ArOH), 8.34 (2 H, s, D2O exch. ArOH); 13C NMR (100 MHz, d6-DMSO) 68.89 (t, (OH)3ArOCH2CH2OAr(OH)3), 104.60, 105.59 (2 d, 4 × ArH), 136.97, 138.34, 139.56, 139.72 (4 s, Cq, (OH)3ArOCH2CH2OAr(OH)3); (HRMS, ESI+) m/z Calcd for C14H15O8 [M + H]+ 311.0761. found 311.0754., calcd for C14H14O8 C 54.20, H 4.55; found: C 53.6, H 4.70.
5,5′-(Ethane-1,2-diylbis(oxy))bis(benzene-5,4,2,1-tetrayl)dodecaethylhexakisphosphate (21)
A mixture of diethyl chlorophosphite (1.33 mL, 7.8 mmol) and N,N-diisopropylethylamine (1.75 mL, 10.0 mmol) was stirred at room temperature in dry CH2Cl2 (10 mL) to give a yellow solution. 5,5′-(Ethane-1,2-diylbis(oxy))dibenzene-1,2,4-triol (20) (310 mg, 1 mmol) was added in small portions and the solid dissolved with the aid of ultrasound within 5-10 min with the solution remaining a yellow colour which was stirred for a further 30 min. The mixture was cooled using dry ice in acetone, then mCPBA (2.58 g, 15 mmol) in CH2Cl2 (25 mL) was added in one portion, the colour went a dark olive green and the solution was stirred for a further 30 min. The mixture was washed with 0.50 M aqueous phosphate buffer pH 7.4 (2 × 100 mL), dried, and was purified by flash chromatography using EtOAc then EtOAc-EtOH (5:1) to give the title compound (21) as a syrup, (809 mg, 72 %), Rf = 0.19 (EtOAc-EtOH, 5:1). 1H NMR (400 MHz, CDCl3) 1.28-1.39 (36 H, 2 t, J = 7.0 Hz, 4 × ArOP(O)(OCH2CH3)2), 4.17-4.31 (24 H, m, 2 × ArOP(O)(OCH2CH3)2), 4.35 (4 H, d, J = 2.0 Hz, −ArOCH2CH2OAr-), 7.13 (2 H, br m, ArH), 7.39 (2 H, br m, ArH); 13C NMR (100 MHz, CDCl3) 15.81, 15.89, 15.97 (3 q, 6 × ArOP(O)(OCH2CH3)2), 64.71, 64.77, 64.82, 64.86, 64.93 (5 t, 6 × ArOP(O)(OCH2CH3)2), 67.80 (t, −ArOCH2CH2OAr-), 107.46, 114.88 (2 d, 4 × ArH), 134.63 (Cq, dd, ArOP(O)(OCH2CH3)2), 136.35 (Cq, d, J = 7.4 Hz, ArOP(O)(OCH2CH3)2), 138.43 (Cq, dd, J = 6.6 Hz, ArOP(O)(OCH2CH3)2), 146.69, 146.75 (Cq, −ArOCH2CH2OAr-); 31P NMR (162 MHz, CDCl3) −7.73 (2 × ArOP(O)(OCH2CH3)2), −7.75 (2 × ArOP(O)(OCH2CH3)2), −8.01 (2 × ArOP(O)(OCH2CH3)2). (HRMS, ESI+) m/z Calcd for C38H69O26P6 [M + H]+ 1127.2497. found 1127.2511; C38H68O26P6 C 40.51, H 6.08; found: C 40.1, H 6.15.
Ethane-1,2-diylbis(oxy))bis(benzene-5,4,2,1-tetrayl)hexakisphosphate (9)
Compound (21) (113 mg, 100 μmol) was dissolved in dry CH2Cl2 (10 mL) and dry 2,4,6-collidine (0.5 mL, 3.84 mmol) was added and the solution was stirred over an atmosphere of nitrogen. Bromotrimethylsilane (1.0 mL, 7.57 mmol) was added and the solution was stirred for 3 days at room temperature (there was some precipitation of collidine derivative). The solvents were evaporated off and the reaction mixture was quenched using a mixed solvent of H2O-(2 M) TEAB (3:1, 4 mL). Compound (9) was purified by ion exchange chromatography using Q-Sepharose Fast Flow and a gradient of triethylammonium bicarbonate, (TEAB) 0→2.0 M and the compound was identified by the Briggs test and eluted at 2.0 M TEAB buffer, (yield, 88 μmol, 88%). 1H NMR (400 MHz, D2O) 4.26 (4 H, s, −ArOCH2CH2OAr-), 7.00, 7.13 (4 H, 2 s, 4 × ArH); 13C NMR (100 MHz, D2O) 68.88 (t, −ArOCH2CH2OAr-), 109.85, 114.71 (2 d, 4 × Ar-H), 137.77 (dd, Cq, C-P, J = 1.4, 6.6 Hz, ArOPO32−), 137.99 (d, C-P, J = 6.6 Hz, ArOPO32−), 139.69 (dd, C-P, J = 1.4, 5.9 Hz), 145.41, 145.47 (2 s, (OPO32−)3ArOCH2CH2OAr(OPO32−)3); 31 P NMR (162 MHz, D2O) −1.68 (2 × ArOPO32−), −1.77 (2 × ArOPO32−), −2.67 (2 × ArOPO32−); (HRMS, ESI−) m/z Calcd for C14H19O26P6 [M − H]− 788.8596. found 788.8617.
3,4-Benzyloxyphenol (24)
3-Chloroperoxybenzoic acid (5.18 g, 30 mmol) was added to a solution of 3,4-dibenzyloxybenzaldehyde (22) (6.37 g, 20 mmol) in dry CH2Cl2 (100 mL) and the mixture was stirred for 22 h. The organic solution was washed with an aqueous solution of 10% sodium metabisulfite (2 × 100 mL) and a saturated aqueous solution of sodium hydrogen carbonate (2 × 100 mL). The organic solution was then evaporated to give an orange/yellow syrup, (Rf = 0.54, formate ester, 23). The syrup was dissolved in a mixed solvent containing MeOH (25 mL) and CH2Cl2 (25 mL) and 5 drops of concentrated HCl were added and the solution was stirred for 90 mins. NaHCO3 (5 g) was added and the solution turned orange/brown. The solvents were evaporated to give the crude product which was dissolved in CH2Cl2 (100 mL) and the grey solid was washed with 0.1 M HCl (2 × 100 mL) and water (100 mL). The organic solution was dried (MgSO4) and the solvents were evaporated. The crude product was purified by flash chromatography (CH2Cl2), Rf = 0.14 (CH2Cl2) to give compound (24) as a white solid, (4.37 g, 71%) (m.p. = 109–110 °C) from EtOAc-hexane. 1H NMR (400 MHz, CD3CN) 4.99, 5.07 (4 H, 2 s, 2 × ArOCH2Ph), 6.30 (1 H, dd, J = 2.9, 8.8 Hz, ArH), 6.52 (1 H, d, J = 2.9 Hz, ArH), 6.64 (1 H, s, D2O exch, ArOH), 6.83 (1 H, d, J = 8.8 Hz, ArH), 7.31-7.46 (10 H, m, 2 × CH2Ph). 13C NMR (100 MHz, CD3CN) 71.35, 72.94 (2 t, 2 × CH2Ph), 103.74, 107.44, 118.04, 118.30 (4 d, CH, Ar), 128.70, 128.73, 128.84, 128.87, 129.31, 129.44 (d, CH, ArOCH2Ph), 138.33, 138.87, 142.88, 150.96, 152.91 (Cq, ArOCH2Ph, ArOCH2Ph, ArOH). (MS, FAB+) Calcd for C20H19O3 [M + H]+ 307.1334; found 307.1340. C20H18O3 C 78.41, H 5.92; found: C 78.4, H 5.94.
1,2-bis(3,4-bis(benzyloxy)phenoxy)ethane (25)
A mixture of 3,4-benzyloxyphenol (24) (3.06 g, 10 mmol) ethylene glycol ditosylate (2.22 g, 6 mmol), K2CO3 (2.76 g, 20 mmol) was heated in dry DMF (100 mL) at 80 °C for 20 h. After this time the reaction was incomplete and a further 0.2 equivalents (0.74 g, 2 mmol) was added and heating continued at 80 °C for a further 17.5 h. The DMF was evaporated and the remaining solid was partitioned between water and CH2Cl2 (100 mL of each). The organic solvent was evaporated and the remaining solid was washed with ether-hexane (150 mL) mixture. The remaining solid was then subject to flash chromatography (CH2Cl2) to give pure compound (25) as a white solid. Yield (1.54 g, 48%), m.p. = 134–135 °C from (CH2Cl2-hexane). 1H NMR (400 MHz, CDCl3) 4.16 (4 H, s, (OBn)2ArOCH2CH2OAr(OBn)2), 5.09, 5.12 (4 H, 2 s, 2 × ArOCH2Ph), 6.40 (2 H, dd, J = 2.7, 8.6 Hz, ArH), 6.62 (2 H, d, J = 2.7 Hz, ArH), 6.86 (2 H, d, J = 8.6 Hz, ArH), 7.31-7.46 (20 H, m, 4 × CH2Ph). 13C NMR (100 MHz, CHCl3) 66.87 (t, (OBn)2ArOCH2CH2OAr(OBn)2), 70.97, 72.48 (2 t, 2 × CH2Ph), 103.59, 105.19, 116.86 (3 d, CH, Ar), 127.26, 127.49, 127.70, 128.80, 128.37, 128.47 (d, CH, ArOCH2Ph), 136.96, 137.52, 143.22, 150.12, 153.80 (Cq, (OBn)2ArOCH2CH2OAr(OBn)2). (MS, FAB+) Calcd for C42H38O6 [M]+ 638.2668; found 638.2735. C42H38O6 C 78.97, H 6.00; found: C 79.0, H 5.98.
4,4′-(ethane-1,2-diylbis(oxy))bis(benzene-1,2-diol) (26)
1,2-bis(3,4-bis(benzyloxy)phenoxy)ethane (25) (1.47 g, 2.30 mmol) was dissolved in warm THF (100 mL). Palladium hydroxide (500 mg, 20%) on carbon was then added and the reaction mixture was stirred under an atmosphere of hydrogen for 17 h. TLC (EtOAc) revealed the disappearance of starting material and a new product Rf = 0.54 for the fully deprotected compound. The solution was filtered over a bed of celite and the solvents were evaporated to yield an off-white greyish solid. Purification of 4,4′-(ethane-1,2-diylbis(oxy))dibenzene-1,2-diol (26) was accomplished by recrystallisation Rf = 0.54, EtOAc). Yield, (506 mg, 79 %), after recrystallisation, m.p. = 179-181 °C (MeOH). 1H NMR (400 MHz, d6-DMSO) 4.06 (4 H, s, (OH)2ArOCH2CH2OAr(OH)2), 6.22 (2 H, dd, J = 2.7, 8.6 Hz, ArH), 6.37 (2 H, d, J = 3.1 Hz, ArH), 6.61 (2 H, d, J = 8.6 Hz, ArH), 8.42, 8.91 (4 H, 2 s, (OH)2ArOCH2CH2OAr(OH)2). 13C NMR (100 MHz, d6-DMSO) 67.48 (t, (OH)2ArOCH2CH2OAr(OH)2), 104.01, 105.22, 116.49 (3 d, CH, Ar), 139.76, 146.29, 152.42 (Cq, (OH)2ArOCH2CH2OAr(OH)2). C14H14O6 C 60.43, H 5.07; found: C 60.1, H 5.08.
Dibenzyl-4,4′-(ethane-1,2-diylbis(oxy))-bis(benzene-4,2,1-triyl)tetrakisphosphate (27)
A mixture of carbon tetrachloride, (1.93 mL, 20 mmol), N,N-diisopropylethylamine (1.46 mL, 8.4 mmol), N,N-dimethylaminopyridine (49 mg, 0.4 mmol) and 4,4′-ethane-1,2-diylbis(oxy))dibenzene-1,2-diol (26) (278 mg, 1 mmol) was stirred for 15 min at −10 °C in acetonitrile (25 mL) and the suspension turned pale green in colour. Dibenzylphosphite (2.66 mL, 12.0 mmol) was then added dropwise over 5 min at −10 °C (dry ice alone) and the mixture was stirred for a further 1 h under N2 with the aid of ultrasound once the phosphorylating reagent was added to give a clear yellow solution. The solvents were evaporated and the remaining yellow syrup was dissolved in dichloromethane (50 mL), washed with water (50 mL), dried, and the title compound was purified by flash chromatography Rf = 0.32 (EtOAc-Petroleum ether (40-60 °C), 2:1), to give the product (27) as a colourless syrup (300 mg, 23 %). 1H NMR (400 MHz, CDCl3) 4.35 (4 H, s, −ArOCH2CH2OAr-), 5.14-5.18 (16 H, m, 4 × ArOP(O)(OCH2Ph)2), 6.73 (2 H, dd, J = 2.7, 9.0 Hz, 2 × ArH), 7.02 (2 H, d, J = 3.0 Hz, 2 × ArH), 7.30-7.35 (42 H, m, 4 × ArOP(O)(OCH2Ph)2, 2 × ArH); 13C NMR (100 MHz, CDCl3) 66.66 (t, −ArOCH2CH2OAr-), 69.84, 69.90, 69.95, 70.01 (4 t, 4 × ArOP(O)(OCH2Ph)2), 107.78, 107.81, 111.68 (3 d, 6 × CH, ArH), 121.95, 121.97 (d, CH, ArH), 127.79, 127.84, 128.36, 128.38, 128.43 (d, CH, ArOP(O)(OCH2Ph)2), 135.11, 135.19, 135.21, 135.25, 135.28, 141.64, 155.72 (s, Cq, (2−O3PO)2ArOCH2CH2OAr(OPO32−)2); 31P NMR (162 MHz, CDCl3) −5.69 (2 × ArOP(O)(OCH2Ph)2), −6.44 (2 × ArOP(O)(OCH2Ph)2), (MS, ES+) Calcd for C70H67O18P4 [M + H]+ 1319.3272; found 1319.3277. calcd for C70H66O18P4 C 63.73, H 5.04; found: C 63.5, H 4.92.
4,4-(Ethane-1,2-diylbis(oxy)bis(benzene-4,2,1-triyl)tetrakisphosphate (10)
The octabenzylphosphate (27) (138 mg, 105 μmol) was dissolved in dry CH2Cl2 (15 mL) and dry 2,4,6-collidine (1.17 mL, 9 mmol) was added and the solution was stirred over an atmosphere of nitrogen. Bromotrimethylsilane (1.0 mL, 7.57 mmol) was added and the solution was stirred for 24 h at room temperature and then a further 2 days since it was difficult to monitor (there was some precipitation of collidine derivative). The solvents were evaporated off and the reaction mixture was quenched using a mixed solvent of H2O-(2 M) TEAB (3:1, 4 mL). Compound (10) was purified by ion exchange chromatography using Q-Sepharose Fast Flow and a gradient of triethylammonium bicarbonate, (TEAB) 0→2.0 M and the compound was identified by the Briggs test and eluted at 1.50-2.0 M TEAB buffer. An impurity due to the incomplete deprotection of the phosphate triester was present. To complete the deprotection the phosphate derivative was dissolved in water and 10% Pd/C (100 mg) was added to the mixture which was stirred over hydrogen for 20 h, yield, (14 μmol, 13%).
1H NMR (400 MHz, D2O) 4.25 (4 H, s, −ArOCH2CH2OAr-), 6.66 (2 H, dd, J = 3.1, 9.0 Hz, ArH), 6.92 (2 H, d, J = 2.7 Hz, ArH), 7.14 (2 H, d, J = 9.0 Hz, ArH), 13C NMR (100 MHz, D2O) 67.41 (t, −ArOCH2CH2OAr-), 108.87, 110.64, 122.63 (3 d, 6 × CH ArH), 138.13 (dd, Cq, C-P, J = 5.9, 6.6 Hz, ArOPO32−), 144.29 (d, C-P, J = 5.1, 6.6 Hz, ArOPO32−), 154.42 (2 s, (OPO32−)3ArOCH2CH2OAr(OPO32−)3); 31P NMR (162 MHz, D2O) −1.90 (2 × ArOPO23), −2.31 (2 × ArOPO23). (HRMS, ESI−) m/z Calcd for C14H17O18P4 [M − H]− 596.9371. found 596.9383.
Biphenyl 3,3′,4,4′,5,5′-hexakisphosphate
Outline spectroscopic data for the final compound are given in the footnote1. Full data will be described elsewhere.
Biological Materials and Methods
Unidirectional 45Ca2+ fluxes InsP3R1-overexpressing L15 cells (Miyawaki, 1990] were seeded in twelve-well clusters (Costar, MA) at a density of 6 × 104 cells per well. Experiments were carried out on confluent cell monolayers, between the 6th and 8th day after seeding. 45Ca2+ unidirectional flux experiments were performed at 30 °C on saponin-permeabilized cells, essentially as previously described [Missiaen, 1992, Parys, 1993]. Permeabilization was for 10 min in a solution containing 120 mM KCl, 30 mM imidazole-HCl (pH 6.8), 2 mM MgCl2, 1 mM ATP, 1 mM EGTA and 40 μg ml−1 saponin. The non-mitochondrial Ca2+ stores were subsequently loaded for 45 min in 120 mM KCl, 30 mM imidazole-HCl (pH 6.8), 5 mM MgCl2, 5 mM ATP, 0.44 mM EGTA, 10 mM NaN3 and 150 nM free 45Ca2+ (28 μCi ml−1). Efflux was initiated by incubation in efflux medium (120 mM KCl, 1 mM EGTA, 30 mM imidazole-HCl pH 6.8) containing thapsigargin (10 μM) and Ca2+ release from the stores was subsequently assessed in efflux medium every 2 min. After 10 min, Ins(1,4,5)P3 or Ca2+ ionophore A23187 (10 μM) were added to the efflux medium for 2 min. Compounds (4, 8, 9 and 10) were added for 2 min before the addition of Ins(1,4,5)P3 and remained present until 2 min after the Ins(1,4,5)P3 addition. As compound (11) induced Ca2+ release by itself, it was applied in the absence of Ins(1,4,5)P3. At the end of the experiment, all 45Ca2+ remaining in the stores was released by incubation with 1 ml of a 2% (w/v) sodium dodecyl sulfate solution for 30 min. Concentration-response curves were fitted using Origin 8.0 (Northampton, MA) software using the Hill equation.
Ins(1,4,5)P3 type I 5-Phosphatase assay
Substrate properties of (4, 8, 9, 10 and 11) against 5-phosphatase were investigated by use of the malachite green phosphatase assay and inhibition of enzyme activity was also evaluated as reported earlier [Vandeput, 2007], but using 1.0 μmol Ins(1,4,5)P3 as substrate.
Results
Chemistry
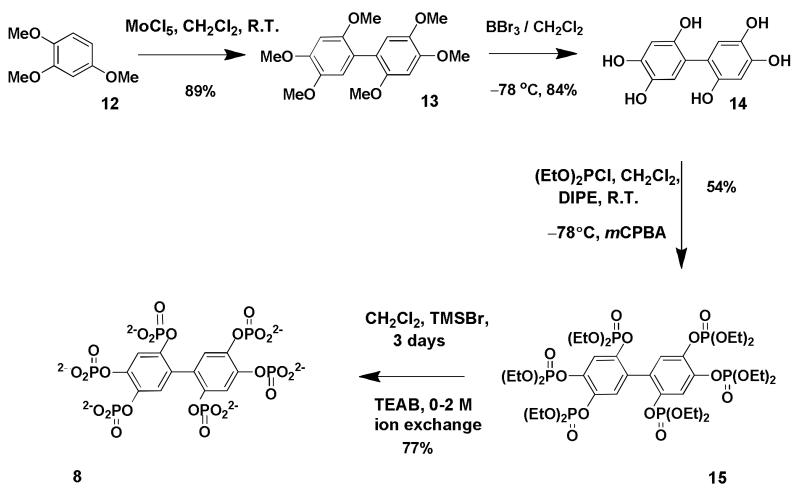
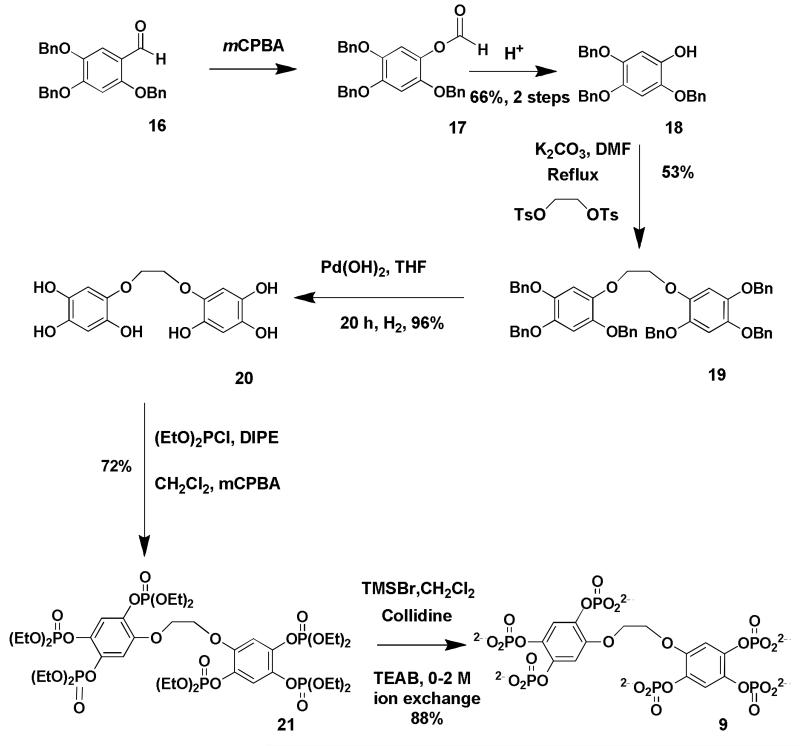
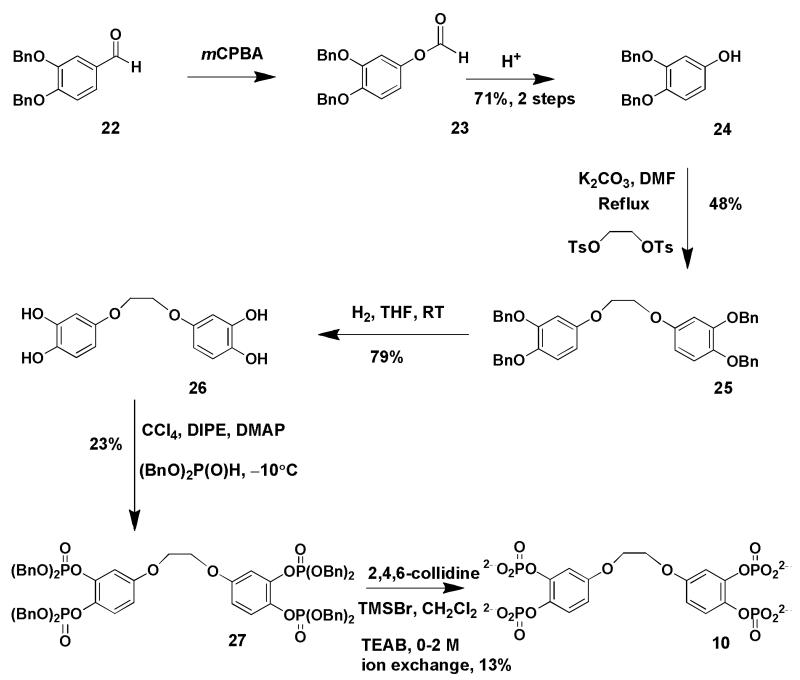
The critical step for the synthesis of compound (8), the more structurally rigid dimer, was the formation of the aryl-aryl bond using the fully protected compound (13). The oxidative coupling reaction was achieved in high yield using molybdenum pentachloride [Waldvogel, 2002] under an atmosphere of argon to give a clean product (13) in high yield. Removal of the methyl groups to expose the six phenolic groups was achieved using excess boron tribromide in dichloromethane. The resulting 2,2′,4,4′,5,5′-hexakisbiphenol (14) is soluble in ethyl acetate and the impurities from the reaction dissolve in the aqueous layer leaving a clean solution of the product. Phosphorylation of six phenolic groups was achieved using P(III) reagent diethoxychlorophosphine followed by oxidation with m-chloroperoxybenzoic acid using a cooling mixture of dry ice and acetone to give the P(V) derivative. This method for introducing the phosphate groups is more efficient than using dibenzyl phosphite, as for the 1,2-phosphorylated dimer as yields are higher and by-products derived from the deprotection of the ethyl groups using bromotrimethylsilane are easy to remove. However, the benzyl protected octabenzylphosphate derivative (27) is easier to identify and purify than the ethylpolyphosphate derivative (15) due to its higher U.V. activity. The critical step for the synthesis of both the ethylene dimers was the coupling of the two aromatic groups via a phenolate to ethylene glycol di-p-tosylate. The potassium phenolate (potassium salt of compounds 18 and 24) doubly displaced both tosyl groups from ethylene glycol di-p-tosylate to provide two aromatic rings linked via an ethylene group (19 and 25 respectively). Since the yields of the displacement were only 50% there was probably elimination of the tosylate competing with the substitution reaction. The full synthesis of compound (11) will be published elsewhere. Compounds (8, 9 and 10) were synthesised according to schemes (1, 2 and 3).
Biology
Effects on InsP3R and on Ca2+ release
Adherent cells form after permeabilization a particularly suitable model system for the investigating the properties of the InsP3R. In this way they allow the loading of the intracellular Ca2+ stores until steady state with 45Ca2+ and the accurate measurement of its unidirectional release under various conditions [Missiaen 1992, Parys 1993a]. This system was used previously for investigating the effects of various pharmacological compounds on Ins(1,4,5)P3-induced Ca2+ release; for example the sulfhydryl reagent thimerosal [Parys 1993b] methylxanthines [Missiaen, 1994] or KN-93 and related compounds [Smyth, 2002].
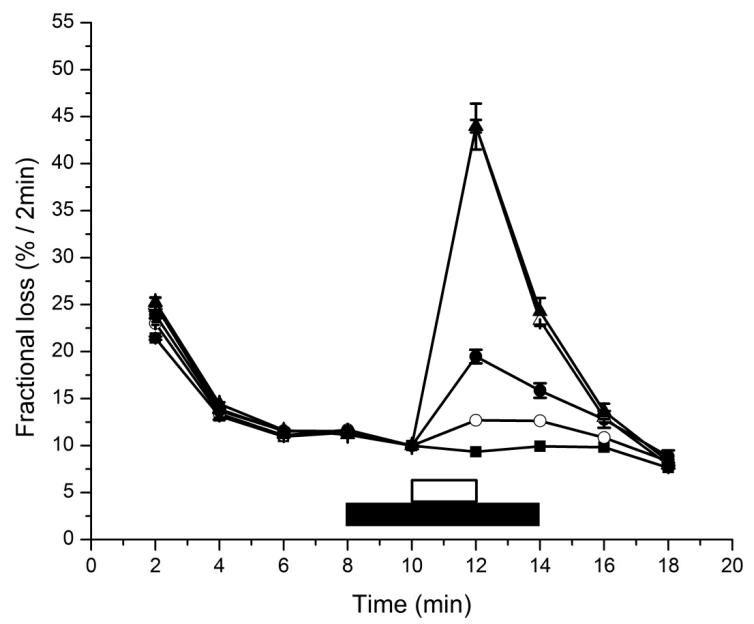
As the InsP3R1 is the most studied isoform, most studies have made use of cell lines predominantly that endogenously express the latter isoform or heterologously express high amounts of it, such as for L15 fibroblasts [Miyawaki 1990]. Saponin-permeabilized L15 fibroblasts have recently been used in a previous study, investigating potential antagonists of the InsP3R [Keddie, 2011]. Figure 4 demonstrates in saponin-permeabilized L15 fibroblasts the effect of low (0.1 μM) and high (3 μM) concentrations of Ins(1,4,5)P3 on unidirectional 45Ca2+ release from the non-mitochondrial Ca2+ stores, whereby the additional inclusion of compound (8) (200 nM) strongly inhibited Ins(1,4,5)P3-induced Ca2+ release at low but not at high concentrations of Ins(1,4,5)P3.
FIGURE 4. Ins(1,4,5)P3-induced Ca2+ release in permeabilized L15 cells.
Fractional Ca2+ loss from non-mitochondrial Ca2+ stores was measured in saponin-permeabilized L15 fibroblasts in the absence of both Ins(1,4,5)P3 and compound (8) (closed squares) or in the presence of 0.1 μM (circles) or 3 μM Ins(1,4,5)P3 (triangles) in the absence (closed symbols) or presence of 200 nM compound (8) (open symbols). EGTA (1 mM) was present throughout the efflux. Fractional loss is defined as the amount of Ca2+ released in 2 min divided by the total store Ca2+ content at that time. Ins(1,4,5)P3 was added for 2 min, as indicated by the white bar. When present, (8) was added to the medium for 6 min, as indicated by the black bar. Results are representative for 3 experiments, each performed in duplicate. The S.D. is indicated, unless it is smaller than the symbol.
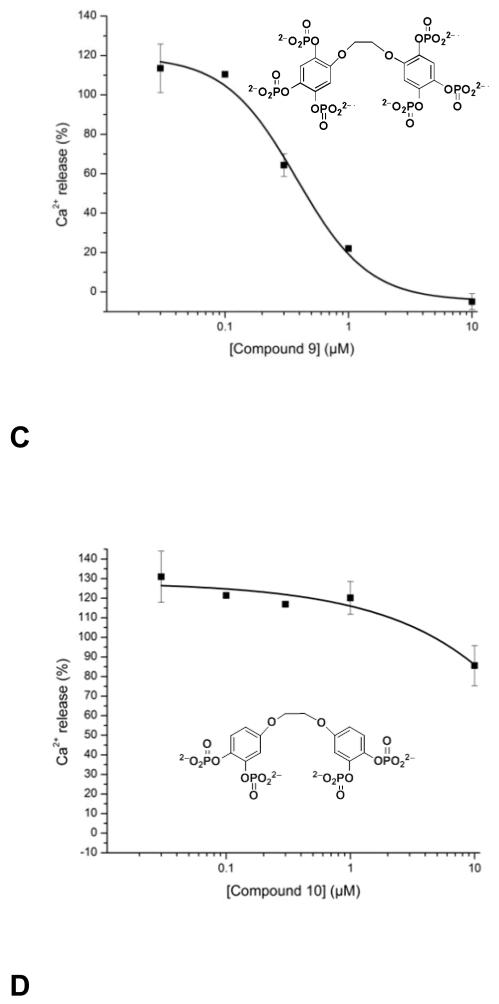
The inhibition observed for compound (8) was further analyzed (Figure 5B) and compared to that obtained by compound (4) (Figure 5A), which was previously investigated [Vandeput, 2007], as well as the effect of the new ligands, (9) (Figure 5C) and (10) (Figure 5D). The various compounds inhibited Ins(1,4,5)P3-induced Ca2+ release in the following rank-order: (8) (IC50 187 ± 21 nM) > (9) (IC50 380 ± 89 nM) ≈ (4) (IC50 417 ± 39 nM) >>> (10) (IC50 >5 μM). These data are collected in Table 1. It was further investigated how the most potent compound (8) acts on the InsP3R by investigating the effect of a fixed concentration (200 nM, its approximate IC50 value) at various Ins(1,4,5)P3 concentrations (Figure 6). From the Lineweaver-Burk plot (Figure 6, inset), (8) appears to act as a competitive inhibitor shifting the apparent Kd for Ins(1,4,5)P3 at the receptor from 400 nM to 900 nM.
FIGURE 5. Effect of compounds 4, 8, 9, and 10 on Ins(1,4,5)P3-induced Ca2+ release.
The concentration-response curves for compounds (4) (panel A), (8) (panel B), (9) (panel C) and (10) (panel D) are shown; Ca2+ release was induced by 250 nM Ins(1,4,5)P3. Measurement of Ins(1,4,5)P3-induced Ca2+ release, incubation with the compounds, and the composition of the efflux medium was exactly as in Figure 4. Ins(1,4,5)P3-induced Ca2+ release was defined as the increase in fractional loss over the basal leak observed after 2 min of incubation with Ins(1,4,5)P3. The Ins(1,4,5)P3-induced Ca2+ release measured in the absence of the compounds was taken as 100%. Each value represents the mean ± S.E. of 3 or 4 experiments, each performed in duplicate.
Table 1. Inhibition of recombinant type I inositol 1,4,5-trisphosphate 5-phosphatase and Ins(1,4,5)P3 - induced Ca2+ release by biphenyl polyphosphates and dimeric benzene polyphosphates.
| Compound | 5-Phosphatase inhibition | Ins P3R antagonism |
|---|---|---|
| IC50 nM | IC50 nM | |
| BiPh(2,3′,4,5′,6)P5 (4) | 1850 | 417 |
| BiPh(2,2′,4,4′,5,5′)P6 (8) | 480 | 187 |
| 1,2,4-Dimer (9) | 3550 | 380 |
| 1,2-Dimer (10) | >10,000 | >5000 |
| BiPh(3,3′,4,4′,5,5′)P6 (11) | 730 | N/A |
1.0 μM Ins(1,4,5)P3 was used as substrate in the 5-phosphatase assay.
FIGURE 6. Compound 8 is a competitive inhibitor of the Ins(1,4,5)P3 receptor.
Ins(1,4,5)P3-induced Ca2+ release was measured at various concentrations of Ins(1,4,5)P3 in the absence (squares) or presence (circles) of 187 ± 21 nM (8). The maximal Ca2+ release, i.e. the release induced by 10 μM of the Ca2+ ionophore A23187, was taken as 100%. The inset shows a Lineweaver-Burk representation of the same data. Fitting was performed by linear regression. (8) Appears to be a competitive inhibitor of the InsP3R, and the apparent Kd for Ins(1,4,5)P3 changes from 400 to 900 nM after inclusion of (8). Each data point represents the mean of 3 or 4 independent experiments, each performed in duplicate. S.E. is indicated, unless smaller than the symbol.
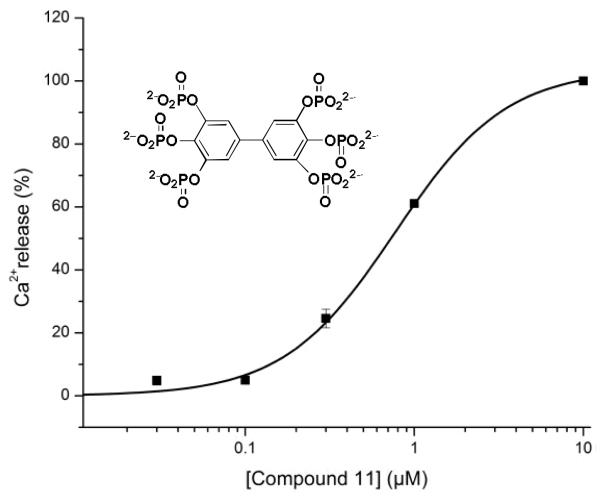
Finally, it is interesting to observe that the related compound (Figure 7) (11), with slightly different phosphate regiochemistry in contrast to the other compounds evaluated, has an effect on the Ca2+ store content in the absence of Ins(1,4,5)P3, inducing Ca2+ release with an EC50 of 800 nM.
FIGURE 7. Compound 11 induces Ca2+ release.
Fractional Ca2+ loss from the non-mitochondrial Ca2+ stores was measured in saponin-permeabilized L15 fibroblasts in the presence of various concentrations of compound (11), EGTA (1 mM) was present throughout the efflux. The Ca2+ release induced by 10 μM (11) was taken as 100%. The experiment was performed in duplicate and S.D. is indicated, unless smaller than the symbol.
The ligands evaluated against InsP3R were also evaluated against type I 5-phosphatase. Table 1 shows three biphenyl compounds (4, 8 and 11) containing five or six phosphate groups spread over two aromatic rings and two dimers (9 and 10) containing six or four phosphate groups respectively, spread over two rings and linked via an ethylene group. In general, the most potent inhibitors are derived from the rigid biphenyl derivatives and the two compounds (8 and 11) containing six phosphates are the most potent inhibitors with IC50 values of less than 1 μM for each. The more flexible dimers are less potent inhibitors of 5-phosphatase, however, the more phosphate groups a molecule contains, the more potent the inhibition of 5-phosphatase in the series.
Discussion
Chemistry
(4) Was previously synthesised and evaluated [Vandeput, 2007]. Compounds (4, 8, 9, 10 and 11) were evaluated at the InsP3R and type I inositol Ins(1,4,5)P3 5-phosphatase. All compounds are resistant to dephosphorylation by 5-phosphatase, as previously demonstrated for (4) and other compounds [Vandeput, 2007].
Bz(1,2,4)P3 (2) can displace [3H-Ins(1,4,5)P3] from bovine adrenal cortex microsomes, but is ca 10,000-fold weaker than Ins(1,4,5)P3 itself [Poitras, 1993]. Although our previous study also shows that Bz(1,2,4)P3 is nearly completely ineffective [Vandeput, 2007], the introduction of another phosphate into the Bz(1,2,4)P3 structure to give benzene 1,2,4,5-tetrakisphosphate nevertheless generates a weak antagonist (IC50 ca 10 μM), the most potent benzene tetrakisphosphate to inhibit Ins(1,4,5)P3 binding to the InsP3R. This provided a clue that increasing the number of phosphate groups around a core could enhance antagonism. Our new synthetic approach to explore this idea towards more potent InsP3R antagonists is to link two aromatic rings and decorate them with phosphate groups. The extended framework of negative charges over a biphenyl motif, for example, could interact with more of the positively charged amino acids in the InsP3R ligand binding site. BiPh(2,3′,4,5′,6)P5 (4) was the first biphenyl polyphosphate derivative that is an InsP3R antagonist and 5-phosphatase inhibitor [Vandeput, 2007] and is used here as a control and compared with the newly synthesized ligands (8) and (11). Derivative (8) comprises a biphenyl core with a relative arrangement of phosphates on each aromatic ring the same as Ins(1,4,5)P3. This core has little flexibility, only rotational mobility and the phosphate groups are more restricted than for Ins(1,4,5)P3 (1). BiPh(3,3′,4,4′,5,5′)P6 (11) is a regioisomer of (8) with one phosphate group on each benzene ring relocated by one carbon atom to form a 1,2,3-trisphosphate motif on both aromatic rings.
The second synthetic approach is to loosely base the core structure of new compounds (9) and (10) on the Ca2+ chelator BAPTA (7), a known weak antagonist of the InsP3R [Morris, 1999]. Ligands were designed in which the two N-methylene carboxylic acids of BAPTA (7) are replaced with two phosphate groups flanking adjacent carbon atoms on the aromatic ring to give compound (10) and introduction of a Bz(1,2,4)P3 (2) motif to give (9) distributes the phosphate groups around the aromatic core in a similar way to those of Ins(1,4,5)P3 (1). Compound (9), containing two copies of (2) joined via a bridged ethylene linker, may also show more potent antagonism at the InsP3R than Bz(1,2,4)P3 since, like biphenyl (8), it possesses a wider spread of phosphate groups than (2) to interact with the positively charged InsP3R ligand binding site.
Biology
Compounds (4 and 8-11) were evaluated in L15 cells overexpressing InsP3R1. Compounds (4, 8 and 9) showed similar shaped concentration-inhibition curves in the presence of 250 nM Ins(1,4,5)P3, where low concentrations of compound (4, 8 and 9 at less than 100 nM for each) have little effect on the amount of Ca2+ released. As the concentration of competing ligand increases in the range from 100 nM to 1000 nM the amount of Ca2+ decreases to nearly zero, demonstrating antagonism of the Ins(1,4,5)P3-induced Ca2+-release. BiPh(2,2′,4,4′,5,5′)P6 (8) with IC50 187 ± 21 nM is the most potent inhibitor of Ca2+ release evaluated, two-fold more potent than either the Bz(1,2,4)P3 dimer (9) IC50 380 ± 89 nM or BiPh(2,3′,4,5′,6)P5 (4), IC50 417 ± 39 nM. The benzene 1,2-bisphosphate dimer (10) is ineffective at inhibiting Ca2+ release up to 10 μM demonstrating, perhaps unsurprisingly, that structurally, two 1,2-bisphosphorylated aromatic rings are insufficient for activity. Dimer (9) is more flexible and slightly longer than biphenyl derivative (8), but the more rigid polyphosphorylated derivative is more potent. The only other compound known with similar inhibition data is a recently reported 5-modified Ins(1,4,5)P3 analogue, where one of the oxygen atoms is replaced with a methyl group [Keddie, 2011] and reported as one of the first examples of an inositol derivative with full antagonistic behavior at the InsP3R. . Under similar experimental conditions application of this analogue at high concentration (300 μM) caused a 40% reduction in the amount of Ins(1,4,5)P3-induced Ca2+ release. The inhibitory effect of BiPh(2,2′,4,4′,5,5′)P6 (8) on the InsP3R is thus much greater than that of this analogue and (8) appears to be a competitive inhibitor of Ins(1,4,5)P3-induced Ca2+-release and shifts the apparent Kd for Ins(1,4,5)P3 from 400 nM to 900 nM. While the data for (8) appear to fit the classical definition of competitive antagonism, more work, eg Schild analysis and competition-binding experiments with labeled Ins(1,4,5)P3 is required for a totally unambiguous classification.
Unlike the inositol ring, the rigid benzene ring cannot alter its shape and phosphate groups attached similarly to a benzene core will have different relative degrees of freedom. The binding domain of the InsP3R is thought to work in a “clam-like” fashion [Rossi, 2009] and Ins(1,4,5)P3, in principle, can also undergo conformational changes. However, phosphorylated biphenyl compounds have in comparison just rotational mobility but little manoeuvrability and flexibility. These effects may also play some part in the antagonistic behavior of these compounds.
The symmetrical 1,2,4-trisphosphate arrangement gives the most potent inhibitor of 5-phosphatase (Table 1) with BiPh(2,2′,4,4′,5,5′)P6 (8) having an IC50 of 480 nM. It is interesting to note for 5-phosphatase inhibition that even though the 1,2,4-trisphosphate arrangement is the same for compounds (8) (IC50 480 nM) and (9) (IC50 3.55 μM) the more rigid and shorter biphenyl polyphosphate (8) is the superior inhibitor.
For 5-phosphatase inhibition, (8) is also ca 4 times better than the original biphenyl polyphosphate synthesized (4) with 5 phosphate groups and regioisomer (11) is intermediate. It is obviously difficult to draw firm SAR conclusions with such a limited compound set but the trend is that potency of the phosphorylated ligands increases (Table 1) with the number of phosphate groups on the aromatic core. Four phosphate groups in a 1,2-bisphosphate arrangement spread over two rings (10) exhibit weaker 5-phosphatase inhibition, whereas (8) is a minimum of 20-fold more potent. For the small set of compounds evaluated the 1,2,4-trisphosphate arrangement around a biphenyl core delivers the most potent ligand.
Regioisomeric BiPh(3,3′,4,4′,5,5′)P6 (11), with a different arrangement of phosphate groups on the aromatic ring compared to (8), was also evaluated. (11) Releases Ca2+ from intracellular stores in a concentration-dependent manner and in the absence of Ins(1,4,5)P3. This is not due to an inhibitory effect on the sarco-/endoplasmic reticulum Ca2+ ATPase (SERCA) pumps as we measure unidirectional efflux of Ca2+ in the presence of thapsigargin, a specific SERCA inhibitor, and EGTA. Therefore, we suppose that (11) might interact with one of the proteins involved in the Ca2+ leak pathway. This pathway is responsible for a continuous leakage of Ca2+ from the stores. The protein(s) responsible for it is(are) however not known with certainty [Sammels, 2010] and possibilities include the translocon complex, polycystin-2, presenilins, Bax-inhibitor-1 and pannexins. Alternatively, it cannot yet be excluded (11) is acting on another type of intracellular Ca2+ release channel such as the cyclic ADP ribose or nicotinic acid-adenine dinucleotide phosphate (NAADP)-sensitive channel [Fliegert, 2007]. This dramatically contrasting effect, elicited by the relocation of only a single phosphate on each phenyl group, is very striking and is worthy of further investigation.
Duchenne muscular dystrophy is an X-chromosome linked muscle wasting disease caused by a defective gene encoding the 427-kDa cytoskeletal protein dystrophin present in muscle [Hoffman, 1987]. A dysfunctional calcium pathway via InsP3Rs releases too much Ca2+ into the skeletal muscle. Interestingly, if the InsP3R is blocked using the non-specific and non-competitive inhibitor 2-aminoethoxydiphenyl borate [Mondin, 2009], there is an improvement of survival of dystrophin-deficient myotubules. The authors suggest targeting the Ins(1,4,5)P3/Ca2+ pathway as a new approach to alleviate symptoms. Understanding the machinery of the InsP3R is thus a priority at both a mechanistic and wider level and intervention may afford leads to target muscular dystrophy and other diseases. Unfortunately, there are few InsP3R antagonists available as leads for such exploitation. Thus, although our new compounds are unlikely to be membrane permeant, we have developed new multivalent ligands with improved antagonist potency and diverse preliminary SAR features. One has been demonstrated to be a competitive antagonist in a functional context and all may be useful tools to investigate the chemical biology of signaling through InsP3R and particularly as templates for further design. Moreover, while a “multivalent” approach has been pioneered for inositol phosphate based ligand design [Riley, 2002, 2004, Rossi, 2009], importantly it now seems clear that this is also applicable for non-inositol polyphosphate ligands, since present data demonstrate a significant enhancement of antagonistic activity for dimers of the very weak Bz(1,2,4)P3 compound achieved in two different ways.
Conclusions
We demonstrate that two new types of dimeric benzene phosphate derivative based on Bz(1,2,4)P3 act as potent sub-micromolar antagonists of Ca2+ release through the InsP3R. Exploration of an earlier structural template based upon a biphenyl core through multivalent considerations has generated an improved antagonist (8) with preliminary SAR considerations and support for a competitive mode of action. Surprisingly, the regioisomeric relative (11) releases Ca2+, and may be interacting with one or more of the proteins connected with the Ca2+ leak pathway. A more flexible multivalent benzene phosphate derivative (9) based loosely around the known weak InsP3R antagonist BAPTA is also more potent than the parent Bz(1,2,4)P3 fragment. The more rigid (8) is the most potent antagonist of Ca2+ release and is also the most potent inhibitor of type I Ins(1,4,5)P3 5-phosphatase, with regioisomer (11) being only slightly weaker. The more rigid structure with the same phosphate arrangement is also a superior 5-phosphatase inhibitor, with flexible derivative (9) being 7-fold weaker than (8). Such templates offer new leads for antagonist design and pharmacological intervention in the polyphosphoinositide pathway of cell signaling.
Scheme 1. Synthesis of biphenyl 2,2′,4,4′,5,5′-hexakisphosphate 8.
Scheme 2. Synthesis of the ethylene-linked benzene 1,2,4-trisphosphate dimer 9.
Scheme 3. Synthesis of ethylene-linked benzene 1,2-bisphosphate dimer 10.
Acknowledgements
We thank the Wellcome Trust (grant number 082837) for Programme Grant support to BVLP. We acknowledge K. Mikoshiba for the generous gift of L15 fibroblasts. This work was supported by Interuniversity Attraction Poles Program P6/28 (Belgian Science Policy) to J.B. Parys and C. Erneux.
Footnotes
Conflict of Interest
The Authors disclose no conflict of interest.
Compound 11 preliminary data: 31P NMR −5.82 (s, 6 P, 6 × ArOPO32−). (HRMS, ESI−) m/z Calcd for C12H14O24P6Na [M +Na – H]− 750.8204. found 750.8215.
References
- Bootman MD, Collins TJ, Macenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Bosanac I, Alattia J-R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Mitsuhiko I. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- Bultynck G, Sienaert I,, Parys JB, Callewaert G, De Smedt H, Boens N, Dehaen W, Missiaen L. Pharmacology of inositol trisphosphate receptors. Pflugers Arch. Eur. J. Physiol. 2003;445:629–642. doi: 10.1007/s00424-002-0971-1. [DOI] [PubMed] [Google Scholar]
- Erneux C, Delvaux A, Moreau C, Dumont JE. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat brain. Biochem. Biophys. Res. Commun. 1986;134:351–358. doi: 10.1016/0006-291x(86)90570-x. [DOI] [PubMed] [Google Scholar]
- Erneux C, Lemos M, Verjans B, Vanderhaeghen P, Delvaux A, Dumont JE. Soluble and particulate Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphatase in bovine brain. Eur. J. Biochem. 1989;181:317–322. doi: 10.1111/j.1432-1033.1989.tb14726.x. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung K-H, Mak D-OD. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegert R, Gasser A, Guse AH. Regulation of calcium signalling by adenine-based second messengers. Biochem, Soc. Trans. 2007;35:109–114. doi: 10.1042/BST0350109. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Takeuchi H, Fleig A, Riley AM, Potter BVL, Hirata M, Penner R. InsP4 facilitates store-operated calcium influx by inhibition of InsP3 5-phosphatase. Nature. 2000;408:735–740. doi: 10.1038/35047115. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Keddie NS, Ye Y, Aslam T, Luyten T, Bello D, Garnham C, Bultynck G, Galione A, Conway SJ. Development of inositol-based antagonists for the D-myo-inositol 1,4,5-trisphosphate receptor. Chem. Commun. 2011;47:242–244. doi: 10.1039/c0cc03003a. [DOI] [PubMed] [Google Scholar]
- Lampe D, Potter BVL. Chemistry of Inositol Lipid Mediated Cellular Signaling. Angew. Chem. Int. edn. Eng. 1995;34:1933–1972. [Google Scholar]
- Lampe D, Liu C, Potter BVL. Synthesis of selective non-Ca2+-mobilising inhibitors of d-myo-inositol 1,4,5-trisphosphate 5-phosphatase. J. Med. Chem. 1994;37:907–912. doi: 10.1021/jm00033a007. [DOI] [PubMed] [Google Scholar]
- Lin C-C, Baek K, Lu Z. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat. Struct. Mol. Biol. 2011;18:1172–1174. doi: 10.1038/nsmb.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Al-Hafidh J, Westwick J, Potter BVL. Synthesis of 1l-chiro-inositol 2,3,5-trisphosphorothioate, the first partial agonist at the platelet myo-inositol 1,4,5-trisphosphate receptor. Bioorg. Med. Chem. 1994;2:253–257. doi: 10.1016/s0968-0896(00)82168-9. [DOI] [PubMed] [Google Scholar]
- Mills SJ, Dozol H, Vandeput F, Backers K, Woodman T, Erneux C, Spiess B, Potter BVL. 3-Hydroxybenzene 1,2,4-Trisphosphate, a novel second messenger mimic and unusual substrate for type I myo-inositol 1,4,5-trisphosphate 5-phosphatase: synthesis and physicochemistry. ChemBioChem. 2006;7:1696–1706. doi: 10.1002/cbic.200600125. [DOI] [PubMed] [Google Scholar]
- Mills SJ, Vandeput F, Trusselle MN, Safrany ST, Erneux C, Potter BVL. Benzene polyphosphates as tools for cell signalling: inhibition of inositol1,4,5-trisphosphate 5-phosphatase and interaction with the PH domain of protein kinase Bα. ChemBioChem. 2008;9:1757–1766. doi: 10.1002/cbic.200800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Droogmans G, Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992;357:599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L,, De Smedt H, Parys JB, Casteels R. Co-activation of inositol trisphosphate-induced Ca2+ releaseby cysosolic Ca2+ is loading-dependent. J. Biol. Chem. 1994;269:7238–7242. [PubMed] [Google Scholar]
- Miyawaki A, Furuichi T, Maeda N, Mikoshiba K. Expressed cerebellar-type inositol 1,4,5-trisphosphate receptor, P400, has calcium release activity in a fibroblast L cell line. Neuron. 1990;5:11–18. doi: 10.1016/0896-6273(90)90029-f. [DOI] [PubMed] [Google Scholar]
- Mondin L, Balghi H, Constantin B, Cognard C, Sebille S. Negative modulation of the inositol 1,4,5-trisphosphate receptor expression prevents dystrophin-deficient muscle death. Am. J. Physiol. Cell Physiol. 2009;297:C1133–C1145. doi: 10.1152/ajpcell.00048.2009. [DOI] [PubMed] [Google Scholar]
- Morris SA, Correa V, Cardy TJA, O’Beirne G, Taylor CW. Interaction between inositol trisphosphate receptors and fluorescent Ca2+ indicators. Cell Calcium. 1999;25:137–142. doi: 10.1054/ceca.1998.0016. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Riley AM, Mills SJ, Lindley CJ, Potter BVL, Westwick J. myo-Inositol 1,4,6-trisphosphorothioate and myo-inositol 1,3,6-trisphosphorothioate: partial agonists with very low intrinsic activity at platelet myo-inositol 1,4,5-trisphosphate receptor. Mol. Pharmacol. 2000;57:595–601. doi: 10.1124/mol.57.3.595. [DOI] [PubMed] [Google Scholar]
- Parys JB, Missiaen L, De Smedt H, Casteels R. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in clonal cell line A7r5. J. Biol. Chem. 1993a;268:25206–25212. [PubMed] [Google Scholar]
- Parys JB, Missiaen L, De Smedt H, Droogmans G, Casteels R. Bell-shaped activation of inositol-1,4,5-trisphosphate-induced Ca2+ release by thimerosal in permeabilized A7r5 smooth-muscle cells. Pflugers Arch. 1993b;424:516–522. doi: 10.1007/BF00374916. [DOI] [PubMed] [Google Scholar]
- Poitras M, Bernier S, Boulay G, Fournier A, Guillemette G. Interaction of benzene 1,2,4-trisphosphate with inositol 1,4,5-trisphosphate receptor and metabolizing enzymes. Eur. J. Pharmacol., Mol. Pharmacol. Section. 1993;244:203–210. doi: 10.1016/0922-4106(93)90145-y. [DOI] [PubMed] [Google Scholar]
- Richardson A, Taylor CW. Effects of Ca2+ on chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J. Biol. Chem. 1993;268:11528–11533. [PubMed] [Google Scholar]
- Riley AM, Morris SA, Nerou EP, Correa V, Potter BVL, Taylor CW. Interactions of inositol 1,4,5-trisphosphate (IP3) receptors with synthetic Poly(ethylene glycol)-linked dimers of IP3 suggest close spacing of the IP3 binding sites. J. Biol. Chem. 2002;277:40290–40295. doi: 10.1074/jbc.M206925200. [DOI] [PubMed] [Google Scholar]
- Riley AM, Laude AJ, Taylor CW, Potter BVL. Dimers of D-myo-Inositol 1,4,5-trisphosphate: design, synthesis and interaction with Ins(1,4,5)P3 receptors. Bioconjugate Chem. 2004;15:278–289. doi: 10.1021/bc034214s. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Riley AM, Tovey SC, Rahman T, Dellis O, Taylor EJA, Veresov VG, Potter BVL, Taylor CW. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 2009;5:631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrany ST, Wilcox RA, Liu C, Dubreuil D, Potter BVL, Nahorski SR. Identification of partial agonists with low intrinsic activity at the inositol-1,4,5-trisphosphate receptor. Mol. Pharmacol. 1993;43:499–503. [PubMed] [Google Scholar]
- Sammels E, Parys JB, Missiaen L, De Smedt H, Bultynck G. Intracellular Ca2+ storage in health and disease: A dynamic equilibrium. Cell Calcium. 2010;47:297–314. doi: 10.1016/j.ceca.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Seo M-D, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Abbott AL, Lee B, Sienaert I, Kasri NN, De Smedt H, Ducibella T, Missiaen L, Parys JB, Fissore RA. Inhibition of the inositol trisphosphate receptor of mouse eggs and A7r5 cells by KN-93 via a mechanism unrelated to Ca2+/calmodulin-dependent protein kinase II antagonism. J. Biol. Chem. 2002;277:35061–35070. doi: 10.1074/jbc.M202928200. [DOI] [PubMed] [Google Scholar]
- Storey DJ, Shears SB, Kirk CJ, Michell RH. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984;312:374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Vandeput F, Combettes L, Mills SJ, Backers K, Wohlkönig A, Parys JB, De Smedt H, Missiaen L, Dupont G, Potter BVL, Erneux C. Biphenyl 2,3′,4,5′,6-pentakisphosphate, a novel inositol polyphosphate surrogate, modulates Ca2+ responses in hepatocytes. FASEB J. 2007;21:1481–1491. doi: 10.1096/fj.06-7691com. [DOI] [PubMed] [Google Scholar]
- Verjans B, De Smedt F, Lecocq R, Vanweyenberg V, Moreau C, Erneux C. Cloning and expression in Escherichia coli of a dog thyroid cDNA encoding a novel inositol 1,4,5-trisphosphate 5-phosphatase. Biochem. J. 1994;300:85–90. doi: 10.1042/bj3000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel SR. The reaction pattern of the MoCl5-mediated oxidative aryl-aryl coupling. Synlett. 2002;4:622–624. [Google Scholar]
- Ward SG, Mills SJ, Liu C, Westwick J, Potter BVL. D-myo-Inositol 1,4,5-trisphosphate analogues modified at the 3-position inhibit phosphatidylinositol 3-kinase. J. Biol. Chem. 1995;270:12075–12084. doi: 10.1074/jbc.270.20.12075. [DOI] [PubMed] [Google Scholar]