Abstract
Introduction
Sudden Infant Death Syndrome (SIDS) remains the leading cause of infant mortality in Western societies. A prior study identified an association between hearing suppression on the newborn hearing test and subsequent death from SIDS. This is the first finding of an abnormality in SIDS cases prior to death. A following study identified that inner ear dysfunction precipitates a marked suppression of the hypercapnic ventilatory response (HCVR). Failure of arousal has been proposed to be a key component in SIDS. The objective of the present study was to assess whether inner ear dysfunction not only weakens the hypercapnic response, but also plays a role in suppressing the arousal response to suffocating gas mixtures.
Methods
Wild-type mice (n = 28) received intra-tympanic gentamicin (IT-Gent) injections bilaterally or unilaterally to precipitate inner ear hair cell dysfunction. Three control groups (n = 22) received intra-tympanic saline (IT-Saline) bilaterally or unilaterally (right or left), or intra-peritoneal gentamicin (IP-Gent). The body movement arousal responses to severe hypoxia–hypercarbia combined (5% CO2 in nitrogen) were tested under light anesthesia 8 days following the administration of gentamicin or saline.
Results
After injections, the bilateral and unilateral IT-Gent-treated animals behaved similarly to controls, however the HCVR as well as the arousal movements in response to severe hypoxia–hypercarbia were suppressed in IT-Gent-treated animals compared to control animals (P < 0.05). Thus the HCVR was significantly decreased in the bilateral (n = 9) and unilateral IT-Gent-treated mice (n = 19) compared to bilateral (n = 7) and unilateral IT-Saline (n = 9) control groups (p < 0.05). Arousal movements were suppressed in the bilateral IT-Gent group (n = 9) compared to bilateral IT-Saline controls (n = 7, P < 0.0001) and in the unilateral IT-Gent group (n = 19) compared to unilateral IT-Saline controls (n = 10, P < 0.0001).
Discussion
The findings support the theory that inner ear dysfunction could be relevant in the pathophysiology of SIDS. The inner ear appears to play a key role in arousal from suffocating gas mixtures that has not been previously identified.
Keywords: inner-ear, gentamicin, vestibular, arousal, severe hypoxia-hypercarbia, SIDS
INTRODUCTION
Sudden Infant Death Syndrome (SIDS) remains the leading cause of postnatal infant mortality in Western societies. It is defined as the unexpected sudden death of a child less than 1 year of age in which an autopsy does not show an explainable cause of death (Hauck and Tanabe, 2008). Although the underlying mechanism has remained elusive; several theories propose failure of respiratory and/or cardiac pathways (Guntheroth, 1995; Horne et al., 2005; Goldwater, 2011; Garcia et al., 2013). Petechiae on autopsy of SIDS cases as well as histological evidence of mild respiratory infection in a high percentage of SIDS victims favor a respiratory-related related mechanism (Guntheroth, 1995; Trachtenberg et al., 2012). Moreover, respiratory tracings and abnormalities in brainstem areas involved with cardio-respiratory control and arousal further support the view that a failure of the infant to arouse from a suffocating hypoxic environment is part of the terminal event in SIDS (Kinney et al., 1995; McNamara et al., 1998; Panigrahy et al., 2000; Rand et al., 2007; Kinney, 2009; Darnall et al., 2010.
The arousal response includes two parts: (a) a respiratory component that increases ventilation (rate and depth) and (b) a defensive body movement component that allows an animal or human infant to physically move away from an environment containing suffocating gas mixtures (Phillipson et al., 1979; Mograss et al., 1994; Heydt et al., 2004). Both components are integral for eliciting an effective arousal to the threat of asphyxia during sleep and can occur without waking the animal or infant (Mograss et al., 1994). Thus, if the airway were to become obstructed (e.g. by soft bedding or a pillow cushion) the body movement component would actively move the head away to access fresh air.
Previous human and animal research supports an integral relationship between inner ear function and respiration (Monahan et al., 2002; Thurrell et al., 2003; Miyamura et al., 2004; Jauregui-Renaud et al., 2005; Allen et al., 2011) and a recent report identified suppressed right sided hearing on the newborn hearing screen test in infants that later died of SIDS when compared to control infants (Rubens et al., 2008). This implies that the involvement of inner ear dysfunction could be a potential contributor to the occurrence of SIDS.
To better understand the potential relationship between SIDS and inner ear dysfunction, we developed an animal model with induced inner ear dysfunction (Allen et al., 2011). Intra-tympanic gentamicin (IT-Gent) injection is an established method for damaging inner ear cochlear and vestibular hair cells (Husmann et al., 1998; Wanamaker et al., 1999; Hoffer et al., 2001; Heydt et al., 2004; Hirvonen et al., 2005; Allen et al., 2011). In a previous study, animals with histologically confirmed inner ear damage following IT-Gent injection displayed a suppressed hypercapnic ventilatory response (HCVR; Allen et al., 2011). The objective of the present study was to verify these findings and to assess whether inner ear injury also decreases the body movement arousal response to suffocating gas mixtures. If this were the case it could be pertinent to the mechanism of SIDS. The hypothesis was that inner ear injury would have no effect on the body movement arousal response.
EXPERIMENTAL PROCEDURES
Wild-type (CD-1) mice of both sexes were studied. The use of the animals and the associated procedures of the study were approved by the Animal Care and Use Committee at Seattle Children’s Research Institute.
Injection procedures
At postnatal day 18, animals underwent a hearing test as outlined in our previous study (Allen et al., 2011). Mice were accepted for the study only if they displayed an adequate response to the acoustic stimulus. Following the hearing test, all accepted animals were anesthetized with 2% isoflurane in room air while breathing spontaneously. Either intra-tympanic (IT) or intra-peritoneal (IP) injections were administered once the animal was unresponsive. The procedure for injections was the same as outlined in our previous project (Allen et al., 2011). For the current project we added unilateral IT-Gentin addition to bilateral IT-Gent. The effect of inner ear injury on the respiratory response to hypercarbia and the body movement arousal response to hypoxia–hypercarbia combined was studied 8 days after initial injection (i.e., postnatal day 26).
Gas composition and anesthesia used during experiments
All experiments were conducted in a small animal plethysmography test chamber (Buxco Halcyon plethysmograph; Wilmington, NC, USA). Chamber temperature was maintained between 26 and 28 °C. Air was defined as gas composition 21% O2 balanced N2. The hypercarbia had a gas composition of 21% O2 5% CO2 balanced N2. The severe hypoxia-hypercarbia gas mixture had a composition of 95% N2 5% CO2.
Both the HCVR and arousal tests were performed with the subject under light anesthesia. To anesthetize the subject, sevoflurane in air was delivered into the chamber via a vaporizer connected to the inflow chamber. The test gas independent of composition passed through the sevoflurane vaporizer and plethysmography chamber throughout the experiment at a continuous rate of 300 ml/min. Sevoflurane concentration was maintained with a final concentration between 1.25% and 1.5% so that the subject was just immobile.
Inspired 1.25–1.5% sevoflurane is substantially less than the 3% needed for surgical procedures (Liao et al., 2006; Koyama et al., 2009; De Segura et al., 2009). Animals were thus only lightly anesthetized and would become conscious within seconds of discontinuing the anesthesia.
HVCR test
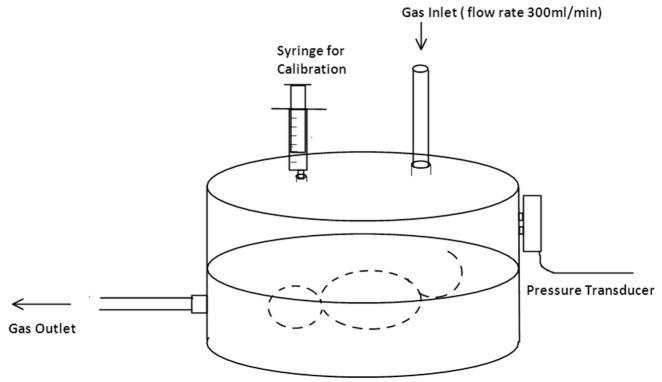
Once respiration had stabilized and the animal was still, the plethysmograph was calibrated by injection of known volumes (0.05 and 0.1 ml) of air into the chamber (Fig. 1). Ventilatory measures were then obtained while the animal was breathing air and then while exposed to the hypercarbia gas mixture for 5 min. Instantaneous respiratory frequency (finst, Hz) and tidal volume (TV, μl) were recorded continuously. Mean minute ventilation (Vmin, ml/min) was calculated continuously as = TV × finst × 60/1000. The HCVR response was reported as a normalized Vmin ratio (nVmin) and was calculated by comparing the peak Vmin recorded during CO2 exposure to Vmin in air. Similarly, finst and TV values at peak Vmin during hypercarbia were compared to the respective metrics in air. These comparisons were also reported as a normalized frequency ratio (nfinst) and normalized TV ratio (nTV).
Fig. 1.
Small animal plethysmography test chamber.
For the HCVR assessments, unilateral and bilateral IT-Gent groups were compared to unilateral and bilateral intra-tympanic saline (IT-Saline) control groups. For the test animals, nine mice were given IT-Gent bilaterally, 19 received unilateral IT-Gent (right 14, left 5). Two control groups were utilized. The first control group (n = 7) received bilateral saline injections intra-tympanically (bilateral IT-Saline). The second control group (n = 10) received a unilateral intra-tympanic injection (unilateral IT-Saline, right 5, left, 5).
Assessment of the body movement arousal responses to severe hypoxia–hypercarbia
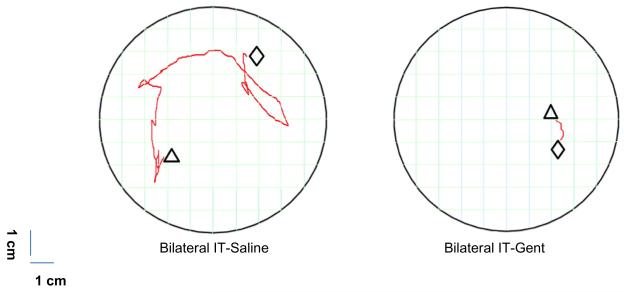
Immediately after testing the HCVR response, the hypercarbia gas mixture was replaced with the severe hypoxia–hypercarbia gas mixture. The response from this point until the end of the experiment was recorded on high definition video using a Lumix DMC-G3 Camera (Panasonic, Osaka, Japan 2011) at a fixed height and distance from the plethysmography chamber. Movements associated with the severe hypoxiac–hypercarbia gas stimulus were defined as “body movement arousal”. The video recording was used to determine the onset time of the first body movements and to measure the total distance moved during the exposure to the severe hypoxia–hypercarbia gas mixture. The severe hypoxia–hypercarbia challenge was terminated when the respiratory rate decreased to eight breaths/minute, at which point air was administered until the animal recovered. If no arousal movement occurred, the time for onset of arousal was taken when the respiratory rate reached 8/min. The main objective was to assess if an animal was able to adequately move its head and body away from the severe hypoxia–hypercarbia stimulus. For the measurement of distance traveled, the center of the ear most visible to the observer was taken for the starting and end point of the head movement in all animals. The pathway of movement was measured on a 10 × 10 grid map with individual square sizes of 1.0 × 1.0 cm (Fig. 2) by two blinded observers. On completion of the body movement arousal assessments animals were immediately euthanized.
Fig. 2.
Representative path of movement (red lines) of individual subjects receiving either bilateral IT-saline (left) or bilateral IT-Gent (right). The grid represents the two dimensional view looking down on the animal in the plethysmography chamber (boundaries of the circle) during the body movement arousal attempt to the anoxia-CO2 stimulus taken from the midpoint of the ear. Triangle: starting point; diamond: end point. All control group animals (IT- Saline, or IP-Saline/IP-Gent) displayed significantly increased arousal movements compared to all IT-Gent test animals. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In addition to the two test and two control groups outlined above for the HCVR assessments we included two additional control groups for the arousal assessments. To control for potential systemic absorption of gentamicin, the third control group (IP-Gent, n = 5) was given an intra-IP injection of 0.05 ml of gentamicin, 8 mg/ml (20% of the gentamicin dose received by the IT-Gent group given bilateral injections). Plasma concentration of gentamicin following intra-tympanic injection is typically undetectable, but has been reported to be up to 4% of the dose per dl (Becvaroski et al., 2002). For the IP-Gent group, we desired to control the possibility of systemic absorption of gentamicin leading to effects unrelated to hair cell damage and therefore injected 20% of the intra-tympanic dose intra-peritoneally in these animals. The fourth control group included five IP-Saline animals to control for the IP procedure and anesthesia that animals received for the injections. (IP control responses were analyzed in our previous study of the HCVR (Allen et al., 2011) therefore for simplification we did not repeat that analysis here).
Statistical analysis
Statistical analysis was performed with GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA). Two-way ANOVA tests were utilized for assessment of the HCVR and arousal responses. Post hoc Bonferroni correction for multiple comparisons was performed between saline and corresponding gentamicin-treated groups with the site of injection (IT or IP). P < 0.05 was accepted for statistical significance. Unless otherwise stated, data were expressed as means ± SE. Statistical comparison was achieved for the HCVR, body arousal movements and time to onset of arousal.
RESULTS
The respiratory response to hypercarbia
Vmin
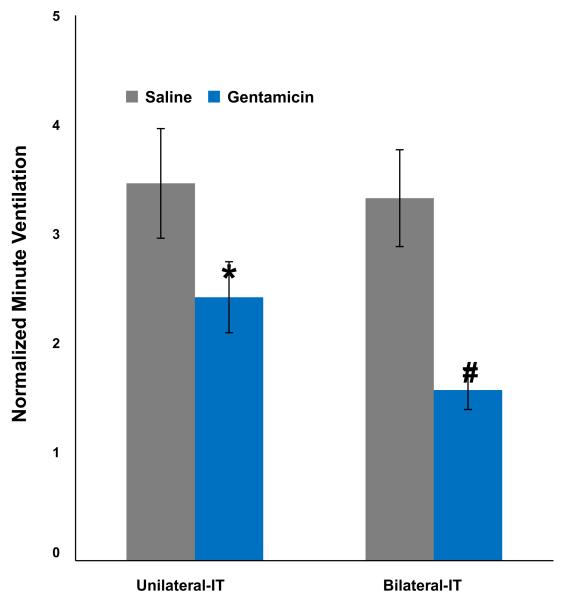
The minute ventilatory response of control animals (unilateral and bilateral IT-Saline groups) increased 3.46 and 3.32-fold in response to hypercarbia compared to baseline in air alone. In contrast, the Vmin response of the unilateral and bilateral IT-Gent test animals was significantly suppressed (30% and 53% decrease respectively, p < 0.05) compared to corresponding control groups (Table 1, Fig. 3). Consequently, the combined changes in respiratory rate and TV (which did not necessarily each reach significance in their own right, see below) resulted in significant suppression of the minute ventilatory responses and therefore HCVR in IT-Gent animals compared to controls (p < 0.05, Fig. 3).
Table 1.
Normalized minute ventilation, respiratory frequency and tidal volume values during the 5 min-exposure to hypercarbia expressed as a fraction of the baseline value (in air alone) and given as mean ± SE (n)
| Intervention | Normalized minute ventilation (Vmin) |
Normalized respiratory frequency (nfinst) |
Normalized tidal volume (nTV) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Saline | Gentamicin |
P value1 |
Saline | Gentamicin |
P value1 |
Saline | Gentamicin | P value1 | |
| Unilateral IT |
3.46 ± 0.50 (9) | 2.42 ± 0.18 (19) | 0.009* | 1.93 ± 0.20 (9) | 1.59 ± 0.10 (19) | 0.075 | 1.84 ± 0.18 (9) | 1.40 ± 0.07 (19) | 0.010* |
| Bilateral IT | 3.32 ± 0.44 (7) | 1.56 ± 0.06 (9) | 0.001* | 1.50 ± 0.26 (7) | 1.26 ± 0.06 (9) | 0.298 | 1.63 ± 0.24 (7) | 1.17 ± 0.07 (9) | 0.062 |
P < 0.05.
P value calculations included post hoc Bonferroni correction indicating whether bilateral and unilateral IT-Gent group differed from corresponding control groups.
Fig. 3.
Normalized minute ventilation (nVmin, mean + SE) during exposure to hypercarbia (ratio of the peak minute ventilation compared to baseline). *The HCVR of the unilateral IT-Gent was significantly suppressed compared to the unilateral IT-Saline control group (p < 0.05). #The HCVR response of the bilateral IT-Gent group was significantly suppressed compared to the bilateral IT-Saline group and also more suppressed than the unilateral IT-Gent group (p < 0.05).
Breakdown of respiratory rate and TV changes (Table 1). Respiratory rate response to hypercarbia
Respiratory rate increased 1.93- and 1.5-fold in the unilateral and bilateral IT-Saline controls compared to baseline. The respiratory rate responses of the unilateral and bilateral IT-Gent groups were relatively suppressed however this did not reach significance when compared to corresponding IT-Saline controls (p > 0.075, 0.298, respectively).
TV
TV responses increased 1.84 and 1.63-fold in the unilateral and bilateral IT-Saline control groups compared to baseline. The TV response was suppressed in the unilateral IT-Gent group compared to the unilateral IT-Saline control group (p < 0.01). The TV response was also suppressed in the bilateral IT-Gent group; however this did not reach significance when compared to the bilateral IT-Saline control group (p > 0.062).
Body movement arousal responses to severe hypoxia–hypercarbia
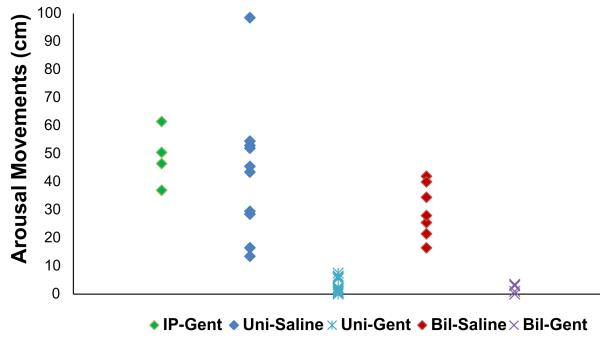
The distance moved (in cm) in response to severe hypoxia–hypercarbia was suppressed in all IT-Gent-treated groups as compared to corresponding IT-Saline control groups (Fig. 4 and Table 2). The difference (P < 0.05) remained when all IT-Gent-treated mice (n = 28) were compared to all controls (n = 22). Arousal was thus significantly suppressed in the bilateral IT-Gent group compared to bilateral IT-Saline controls (P < 0.0001) and in the unilateral IT-Gent group compared to unilateral IT-Saline controls (P < 0.0001). Unilateral and bilateral IT-Gent animals did not differ significantly in their responses and no significant difference was seen between the IT-saline groups and the IP-Gent group. These data indicate that IT injection whether bilateral or unilateral significantly suppressed arousal movements compared to the respective IT-saline control group. There was no significant difference in body arousal movements between the IP-Gent and IP-Saline control groups (p > 0.05, Table 2).
Fig. 4.
Distance of arousal movements in response to severe hypoxia–hypercarbia stimulus in unilateral and bilateral IT-Gent-treated animals compared to corresponding IT-Saline and IP-Gent control groups. Data show individual animal responses. Bil: bilateral IT; Uni: unilateral IT; IT: intra-tympanic injection; IP: intra-peritoneal injection. For numerical data, see Table 2.
Table 2.
Movement in centimeters during the arousal response to severe hypoxia–hypercarbia (Fig. 4). IT: intra-tympanic. IP: intra-peritoneal injection. P-values were adjusted for multiple comparisons
| Intervention | Saline |
Gentamicin |
Bonferroni multiple comparisons P value | ||
|---|---|---|---|---|---|
| Mean ± SE | N | Mean ± SE | N | ||
| Systemic IP | 45.5 ± 5.6 | 5 | 49.2 ± 3.9 | 5 | >0.05 |
| Unilateral IT | 43.5 ± 7.7 | 10 | 2.0 ± 0.6 | 19 | <0.001 |
| Bilateral IT | 29.7 ± 3.6 | 7 | 1.6 ± 0.5 | 9 | <0.0001 |
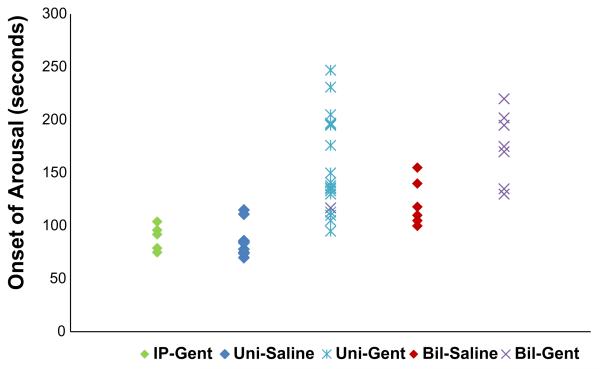
Time to onset of the body movement arousal response
The bilateral and unilateral IT-Gent versus IT-Saline control responses are presented in Fig. 5 and Table 3. As compared to corresponding control groups, the onset time was significantly delayed in the bilateral and unilateral IT-Gent groups. The bilateral IT-Saline control group had a delayed onset of arousal as compared to other control groups (P < 0.05). Thus IT injection whether bilateral or unilateral significantly delayed arousal compared to controls that received IT-Saline. There was also no significant difference in arousal onset time between the IP-Gent and IP-Saline groups (p > 0.05, Table 3).
Fig. 5.
Arousal onset time in response to severe hypoxia–hypercarbia stimulus in unilateral and bilateral IT-Gent-treated animals compared to corresponding IT-Saline and IP-Gent control groups. Data show individual animal responses. Bil: bilateral IT; Uni: unilateral IT; IT: intra-tympanic injection; IP: intra-peritoneal injection. For numerical data, see Table 3.
Table 3.
Time to onset of arousal in seconds (mean ± SE) after commencement of the severe hypoxia–hypercarbia stimulus (Fig. 5). IT: intra-tympanic. IP: intra-peritoneal injection
| Intervention | Saline |
Gentamicin |
Bonferroni multiple comparisons P value | ||
|---|---|---|---|---|---|
| Mean ± SE | N | Mean ± SE | N | ||
| Systemic IP | 85 ± 5 | 5 | 89 ± 5 | 5 | >0.05 |
| Unilateral IT | 85 ± 5 | 10 | 156 ± 10 | 19 | <0.001 |
| Bilateral IT | 120 ± 8 | 7 | 164 ± 12 | 9 | <0.01 |
DISCUSSION
The findings indicate that the inner ear appears to play an integral function in the ability to arouse and physically escape from suffocating gas mixtures. The study also confirmed our previous finding (Allen et al., 2011) that gentamicin-induced inner ear injury decreases the ventilatory response to hypercarbia. This fits well with previous studies in human subjects and animals. The vestibular organ has been identified to have an established role in respiratory control (Morinaka and Nakamura, 1998; Monahan et al., 2002; Thurrell et al., 2003; Miyamura et al., 2004; Jauregui-Renaud et al., 2005; Arshian et al., 2007; Green et al., 2008) as well as autonomic and cardiovascular regulation (Jian et al., 2005; Yates and Bronstein, 2005).
A disturbance of the inner ear that includes the auditory system could have dramatic effects on arousal and the autonomic response to severe hypoxia–hypercarbia (Harper, 2001; Yates and Bronstein, 2005). In addition, neuropathological evidence from SIDS cases supports a significant role for vestibular structures in the fatal outcome of the syndrome (Harper, 2001; Machaalani et al., 2009; Kinney, 2009).
We did not study neuronal activity and can therefore only hypothesize regarding the mechanism whereby vestibular injury would ablate the body movement arousal response to suffocating gas mixtures. For balance adjustments, it is recognized that the vestibular hair cells on the side toward which the head is turning are depolarized, those on the other side are hyperpolarized (Ganong, 1999; Purves et al., 2001). We postulate that severe hypoxia–hypercarbia could elicit a unified depolarization response in vestibular afferents on both sides. This would distinguish it from a balance stimulus and precipitate an ‘unadjusted’ body movement during sleep that would allow the animal to escape suffocation. In fact, evidence supports anoxic and ischemic insult precipitating a depolarization response in specialized sensory neurons, including auditory and vestibular pathways (Burke, 1993; Fern and Ransom, 1997; Kiernan and Bostock, 2000; Lin et al., 2001; Hildebrand et al., 2004; Krishnan et al., 2005; Mercado et al., 2006; Ugawa et al., 2006; Zhang et al., 2008a,b). In the presence of even unilateral vestibular organ damage there could be an insufficient number of functioning vestibular neurons available to depolarize and precipitate the movement arousal response. This coincides with our observation that a number of unilateral IT-Gent animals appeared to make small ineffective leg movements as if attempting to arouse, yet the body did not move from its original position.
Earlier observations corroborate that the present findings are indeed due to inner ear injury and not from insult to surrounding neuronal structures. In a previous and similar study that utilized the same procedure for injections, we observed a twenty-three fold decrease in the number of hair cells in the vestibular organ of IT-Gent mice compared to IT-saline controls (Allen et al., 2011). In five vestibular nuclei and the dorsal and ventral cochlear nuclei of the brainstem, the neuronal counts and the degree of glial fibrillary acidic protein staining (GFAP, a marker of neuronal degeneration) did not differ from those of control animals. Apoptosis was not observed in any nuclei. It is also noteworthy that high concentrations of gentamicin do not cause brainstem or cerebral toxicity (Becvaroski et al., 2002; Perletti et al., 2008). Furthermore, intra-ventricular gentamicin is regularly utilized to treat meningitis without deleterious effect on brainstem or cerebral function (Ragel et al., 2006). Finally, one would perhaps expect some other behavior changes if IT-Gent did cause brainstem dysfunction. This, however, was neither the case in the previous nor the current project. The mice displayed no behavioral abnormalities in their daily activities and were indistinguishable from untreated siblings or controls.
Some other findings warrant discussion. The body movement arousal response was similarly suppressed in animals with unilateral injuries compared to those with bilateral injuries. This suggests that a one-sided inner ear injury might be adequate to increase the risk of SIDS.
It is noteworthy, that although the HCVR in unilateral IT-Gent animals was significantly suppressed (by 30%) compared to unilateral IT-Saline controls, the bilateral IT-Gent animals displayed even greater HCVR suppression (53% reduction) compared to controls (Fig. 3). Thus the HCVR of unilateral IT-Gent animals appears to lie ‘in between’ the severely suppressed response of the bilateral IT-Gent animals and the robust HCVR seen in control animals. Consequently, this observation suggests that the relationship between suppressed arousal and suppressed HCVR from IT-Gent is complex and may in fact even be independent. The interplay between these two processes clearly deserves scrutiny in future studies.
The arousal onset was also delayed in the bilateral IT-Saline group as compared to the unilateral IT-Saline groups (table 3). This could indicate that IT-Saline causes some disruption of inner ear function and that this injury is more obvious after bilateral injections. If so, one would also expect less arousal movement in the bilateral IT-Saline mice and perhaps also a more delayed response in the bilateral IT-Gent group than in the unilateral IT-Gent groups. This however was not the case.
Study limitations
Measurement limitations included the absence of invasive arterial CO2 monitoring. The hypercarbia and severe hypoxia–hypercarbia challenges were closely monitored and timed, however because of the arousal movement assessment, arterial CO2 and O2 values could not be obtained.
Another consideration is that anesthesia, even at low concentration, is not equivalent to natural sleep. SIDS occurs predominantly during sleep (Harper, 2001). Our aim was to create a state of unconsciousness from which a subject could reliably be aroused after severe hypoxia–hypercarbia stimulus.
It is important to keep in mind that suppression of arousal in IT-Gent animals, and perhaps also SIDS cases (Rubens et al., 2008), may occur at least in part from a hearing deficit. To prevent sound stimuli from contributing to the arousal response, extraneous sounds were carefully controlled during the experiments. There was a barely audible continuous noise from the gas delivery system. The noise changed pitch when the gas mixtures were changed but arousal responses did not occur at this time in Control or IT-Gent animals.
Potential application to SIDS
The peak incidence for SIDS occurs at an age of 2–4 months (Guntheroth, 1995). In mice, this would correspond to an age of approximately 8–12 days (P8–12). (Cummings et al., 2011). At that age, we could not reliably access the tympanic membrane through the ear canal to perform the injections. Although it seems unlikely that younger mice would be more resistant to inner ear injury, it should thus be noted that the model is not entirely age appropriate. Nevertheless, the present animal model has characteristics that seem to correspond well with SIDS cases. Similar to SIDS infants, IT-Gent animals are indistinguishable morphologically and in their general waking behavior from others (Guntheroth, 1995). Additionally, a decreased arousal response to severe hypoxia–hypercarbia could increase the risk of sudden death during sleep. Notably, autopsy of SIDS cases that included temporal bone analysis have demonstrated bleeding and inflammation in the inner ear (Josset et al., 1985) that could be associated with inner ear dysfunction. Although SIDS could fit well with an inner ear injury, this does not exclude other mechanisms. A predisposing insult could precipitate inner ear as well as brainstem dysfunction. SIDS cases display serotonergic deficits on postmortem analysis of the vestibular nuclei as well as other areas of the brainstem (Kinney et al., 1995; Panigrahy et al., 2000; Kinney, 2009; Machaalani et al., 2009).
Implications for future studies
In our experiments, control animals instigated vigorous arousal movements. In another setting this response could have physically removed them from a suffocating environment. In contrast, IT-Gent animals would remain in the asphyxiating surroundings due to an impaired arousal response. This would predispose individuals with inner ear injury to repeated episodes of hypoxic insult with further blunting of the arousal response. This coincides with the finding that SIDS victims are often exposed to repeated episodes of hypoxia before death (Rognum and Saugstad, 1991; Guntheroth, 1995; Prandota, 2004; Darnall et al., 2010). We therefore plan to study the effect of repeated bouts of hypoxic gas mixtures with and without hypercarbia in IT-Gent animals under natural sleep conditions.
CONCLUSION
Suppression of the body movement arousal response to severe hypoxia–hypercarbia stimulus from unilateral or bilateral inner ear insult is an important finding. The inner ear appears to play a key role in respiratory control and arousal from suffocating gas mixtures that has not been previously identified. Further studies are needed to elucidate how the inner ear affects respiratory control and the arousal response and to determine if these mechanisms can explain sudden infant deaths.
Acknowledgements
Department of Anesthesia/ITHS Pilot grant. Grateful to Christer Jonmarker, Philip Morgan, Jerry Kim, Lynn Martin and Saritha sankarankutty for their input, editing and moral support.
Abbreviations
- finst
instantaneous respiratory frequency
- HCVR
hypercapnic ventilatory response
- IP-Gent
intra-peritoneal gentamicin
- IP-Saline
intra-peritoneal saline
- IT-Gent
intra-tympanic gentamicin
- IT-Saline
intra-tympanic saline
- nTV
normalized tidal volume
- SIDS
Sudden Infant Death Syndrome
- TV
tidal volume
- Vmin
minute ventilation
REFERENCES
- Allen T, et al. Inner ear insult suppresses the respiratory response to carbon dioxide. Neuroscience. 2011;175:262–272. doi: 10.1016/j.neuroscience.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Arshian M, Holtje RJ, Cotter LA, Rice CD, Cass SP, Yates BJ. Consequences of postural changes and removal of vestibular inputs on the movement of air in and out of the lungs of conscious felines. J Appl Physiol. 2007;103(1):347–352. doi: 10.1152/japplphysiol.00211.2007. [DOI] [PubMed] [Google Scholar]
- Becvaroski Z, Bojrab DI, Michaeilides EM, Kartush JM, Zappia JJ, LaRouere MJ. Round window gentamicin absorption: an in vivo human model. Laryngoscope. 2002;112(9):1610–1613. doi: 10.1097/00005537-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Burke D. Microneurography, impulse conduction, and paresthesias. Muscle Nerve. 1993;16(10):1025–1032. doi: 10.1002/mus.880161005. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol. 2011;111(3):825–833. doi: 10.1152/japplphysiol.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, McWilliams S, Schneider RW, Tobia CM. Reversible blunting of from sleep in response to intermittent hypoxia in the developing rat. J Appl Physiol. 2010;109(6):1686–1696. doi: 10.1152/japplphysiol.00076.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Segura IA, de la Víbora JB, Criado A. Determination of the minimum alveolar concentration for halothane, isoflurane and sevoflurane in the gerbil. Lab Anim. 2009;43(3):239–242. doi: 10.1258/la.2008.006065. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR. Ischemic injury of optic nerve axons: the nuts and bolts. Clin Neurosci. 1997;4(5):246–250. [PubMed] [Google Scholar]
- Ganong WF. Review of medical physiology. nineteenth edition Appleton and Lange; 1999. p. 174. [Google Scholar]
- Garcia AJ, 3rd, Koschnitzky JE, Ramirez JM. The physiological determinants of sudden infant death syndrome. Respir Physiol Neurobiol. 2013 doi: 10.1016/j.resp.2013.05.032. http://dx.doi.org/10.1016/j.resp.2013.05.032. pii: S1569-9048(13)00182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater PN. A perspective on SIDS pathogenesis. The hypotheses: plausibility and evidence. BMC Med. 2011;9:64. doi: 10.1186/1741-7015-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DA, Golding JF, Aulakh M, Faldon MC, Murphy KG, Bronstein AM, Gresty MA. Adaptation of ventilation to ‘buffeting’ in vehicles. Clin Auton Res. 2008;18(6):346–351. doi: 10.1007/s10286-008-0491-y. [DOI] [PubMed] [Google Scholar]
- Guntheroth W. The sudden infant death syndrome. third edition Futura Publishing Company; 1995. [Google Scholar]
- Harper RM. Autonomic control during sleep and risk for sudden death in infancy. Arch Ital Biol. 2001;139(3):185–194. [PubMed] [Google Scholar]
- Hauck FR, Tanabe KO. International trends in sudden infant death syndrome: stabilization of rates requires further action. Pediatrics. 2008;122(3):660–666. doi: 10.1542/peds.2007-0135. [DOI] [PubMed] [Google Scholar]
- Heydt JL, Cunningham LL, Rubel EW, Coltrera MD. Round window gentamicin application: an inner ear hair cell damage protocol for the mouse. Hear Res. 2004;192(1-2):65–74. doi: 10.1016/j.heares.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hildebrand MS, de Silva MG, Klockars T, Rose E, Price M, Smith RJ, McGuirt WT, Christopoulos H, Petit C, Dahl HH. Characterisation of DRASIC in the mouse inner ear. Hear Res. 2004;190:149–160. doi: 10.1016/S0378-5955(04)00015-2. [DOI] [PubMed] [Google Scholar]
- Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Allen K, Kopke RD, Weisskopf P, Gottshall K, Wester D. Transtympanic versus sustained-release administration of gentamicin: kinetics, morphology, and function. Laryngoscope. 2001;111(8):1343–1357. doi: 10.1097/00005537-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Horne RS, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir Physiol Neurobiol. 2005;149(1-3):257–271. doi: 10.1016/j.resp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Husmann KR, Morgan AS, Girod DA, Durham D. Round window administration of gentamicin: a new method for the study of ototoxicity of cochlear hair cells. Hear Res. 1998;125(1-2):109–119. doi: 10.1016/s0378-5955(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Jauregui-Renaud K, Villanueva PL, del Castillo MS. Influence of acute unilateral vestibular lesions on the respiratory rhythm after acute change in posture in human subjects. J Vestib Res. 2005;15(1):41–48. [PubMed] [Google Scholar]
- Jian BJ, Acernese AW, Lorenzo J, Card JP, Yates BJ. Afferent pathways to the region of the vestibular nuclei that participates in cardiovascular and respiratory control. Brain Res. 2005;1044(2):241–250. doi: 10.1016/j.brainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Josset P, Lecomte D, Omur M, Chabolle F, Hannoun L, Chouard CH. Anatomo-pathologic study of the temporal bone in our 4 cases of sudden infant death. Ann Otolaryngol Chir Cervicofac. 1985;102(6):425–432. [PubMed] [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123(Pt 12):2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51(3):223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;8:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Koyama T, Mayahara T, Wakamatsu T, Sora I, Fukuda K. Deletion of mu-opioid receptor in mice does not affect the minimum alveolar concentration of volatile anaesthetics and nitrous oxide-induced analgesia. Br J Anaesth. 2009;103(5):744–749. doi: 10.1093/bja/aep246. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Kiernan MC. Excitability differences in lower-limb motor axons during and after ischemia. Muscle Nerve. 2005;31(2):205–213. doi: 10.1002/mus.20258. [DOI] [PubMed] [Google Scholar]
- Liao M, Laster MJ, Eger EI, 2nd, Tang M, Sonner JM. Naloxone does not increase the minimum alveolar anesthetic concentration of sevoflurane in mice. Anesth Analg. 2006;102(5):1452–1455. doi: 10.1213/01.ane.0000204254.87933.f6. [DOI] [PubMed] [Google Scholar]
- Lin CS, Mogyoros I, Kuwabara S, Cappelen-Smith C, Burke D. Differences in responses of cutaneous afferents in the human median and sural nerves to ischemia. Muscle Nerve. 2001;24(11):1503–1509. doi: 10.1002/mus.1175. [DOI] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA. Serotoninergic receptor 1A in the sudden infant death syndrome brainstem medulla and associations with clinical risk factors. Acta Neuropathol. 2009;117(3):257–265. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85(6):2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- Mercado F, López IA, Acuna D, Vega R, Soto E. Acid-sensing ionic channels in the rat vestibular endorgans and ganglia. J Neurophysiol. 2006;96(3):1615–1624. doi: 10.1152/jn.00378.2006. [DOI] [PubMed] [Google Scholar]
- Miyamura M, Ishida K, Katayama K, Shima N, Matsuo H, Sato K. Ventilatory and heart rate responses at the onset of chair rotation in man. Jpn J Physiol. 2004;54(5):499–503. doi: 10.2170/jjphysiol.54.499. [DOI] [PubMed] [Google Scholar]
- Mograss MA, Ducharme FM, Brouillette RT. Movement/arousals. Description, classification, and relationship to sleep apnea in children. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1690–1696. doi: 10.1164/ajrccm.150.6.7952634. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Sharpe MK, Drury D, Ertl AC, Ray CA. Influence of vestibular activation on respiration in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R689–R694. doi: 10.1152/ajpregu.00568.2001. [DOI] [PubMed] [Google Scholar]
- Morinaka S, Nakamura H. Arterial blood gas abnormalities in patients with dizziness. Ann Otol Rhinol Laryngol. 1998;107(1):6–9. doi: 10.1177/000348949810700102. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59(5):377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Perletti G, Vral A, Patrosso MC, Marras E, Ceriani I, Willems P, Fasano M, Magri V. Prevention and modulation of aminoglycoside ototoxicity (Review) MolMed Rep. 2008;1(1):3–13. [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE. Arousal, the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1979;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- Prandota J. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther. 2004;11(6):517–546. doi: 10.1097/01.mjt.0000140648.30948.bd. [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, et al. Neuroscience. 2nd edition. Sinauer Associates; Sunderland (MA): 2001. The Semicircular Canals. [Google Scholar]
- Ragel BT, Browd SR, Schmidt RH. Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg. 2006;105(2):242–247. doi: 10.3171/jns.2006.105.2.242. [DOI] [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Sudden infant death syndrome: rare mutation in the serotonin system FEV gene. Pediatr Res. 2007;62:180–182. doi: 10.1203/PDR.0b013e3180a725a0. [DOI] [PubMed] [Google Scholar]
- Rognum TO, Saugstad OD. Hypoxanthine levels in vitreous humor: evidence of hypoxia in most infants who died of sudden infant death syndrome. Pediatrics. 1991;87(3):306–310. [PubMed] [Google Scholar]
- Rubens DD, Vohr BR, Tucker R, O’Neil CA, Chung W. Newborn oto-acoustic emission hearing screening tests: preliminary evidence for a marker of susceptibility to SIDS. Early Hum Dev. 2008;84(4):225–229. doi: 10.1016/j.earlhumdev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Thurrell A, Jáuregui-Renaud K, Gresty MA, Bronstein AM. Vestibular influence on the cardiorespiratory responses to whole-body oscillation after standing. Exp Brain Res. 2003;150(3):325–331. doi: 10.1007/s00221-003-1422-8. [DOI] [PubMed] [Google Scholar]
- Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of back-to-sleep campaign. Pediatrics. 2012;129(4):630–638. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa S, Inagaki A, Yamamura H, Ueda T, Ishida Y, Kajita K, Shimizu H, Shimada S. Acid-sensing ion channel-1b in the stereocilia of mammalian cochlear hair cells. NeuroReport. 2006;17(12):1235–1239. doi: 10.1097/01.wnr.0000233093.67289.66. [DOI] [PubMed] [Google Scholar]
- Wanamaker HH, Slepecky NB, Cefaratti LK, Ogata Y. Comparison of vestibular and cochlear ototoxicity from transtympanic streptomycin administration. Am J Otol. 1999;20(4):457–464. [PubMed] [Google Scholar]
- Yates BJ, Bronstein AM. The effects of vestibular system lesions on autonomic regulation: observations, mechanisms, and clinical implications. J Vestib Res. 2005;15(3):119–129. [PubMed] [Google Scholar]
- Zhang M, Gong N, Lu YG, Jia NL, Xu TL, Chen L. Functional characterization of acid-sensing ion channels in cultured neurons of rat inferior colliculus. Neuroscience. 2008a;154(2):461–472. doi: 10.1016/j.neuroscience.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rajan I, Savelieva KV, Wang CY, Vogel P, Kelly M, Xu N, Hasson B, Jarman W, Lanthorn TH. Netrin-G2 and netrin-G2 ligand are both required for normal auditory responsiveness. Genes Brain Behav. 2008b;7(4):385–392. doi: 10.1111/j.1601-183X.2007.00361.x. [DOI] [PubMed] [Google Scholar]