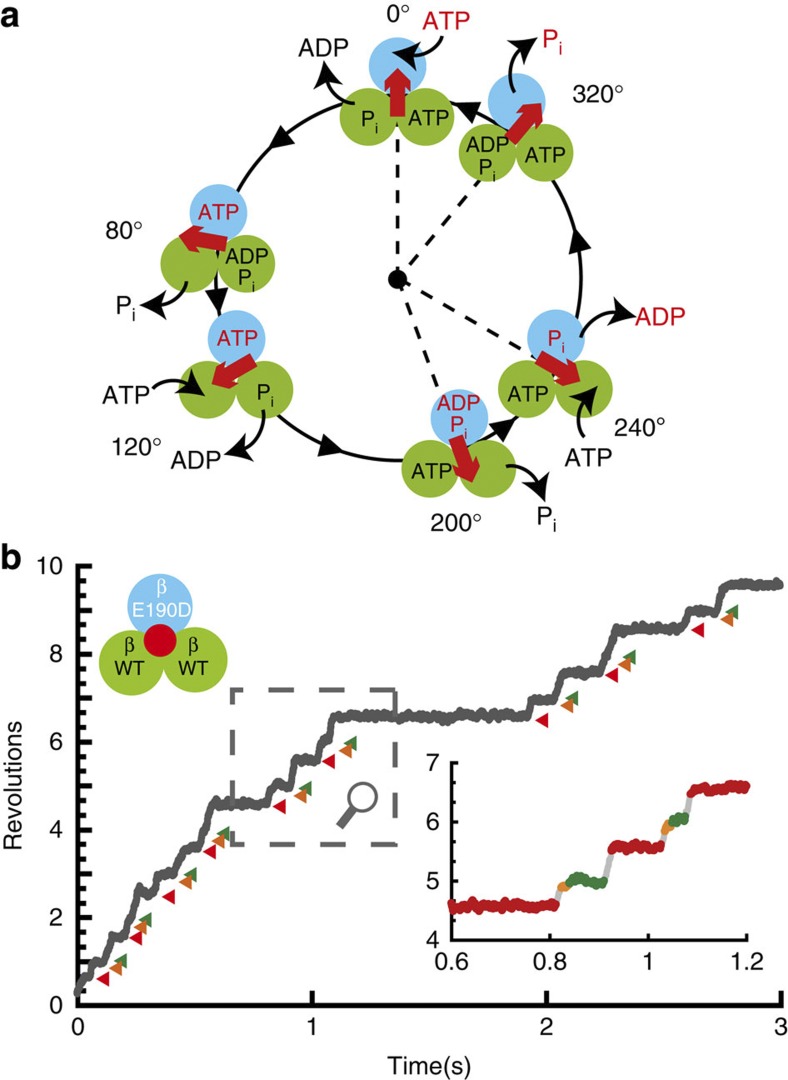
Figure 1. Chemomechanical coupling scheme of F1-ATPase.
(a) The circles and red arrows represent the catalytic state of the β subunits and the angular positions of the γ subunit. Each β subunit completes one turnover of ATP hydrolysis in a turn of the γ subunit, where the three β subunits vary in their catalytic phase by 120°. Regarding the catalytic state of the top β subunit (cyan), ATP binding, hydrolysis, ADP release and inorganic phosphate (Pi) release occur at 0°, 200°, 240° and 320°, respectively. (b) Time course of the rotation of the hybrid F1-ATPase, α3β(E190D) β2γ, in the presence of 1 mM ATP. Red, orange, and green represent the pause at 200°, 320°, and 360° for β(E190D), respectively. The inset shows a magnification of the time course.