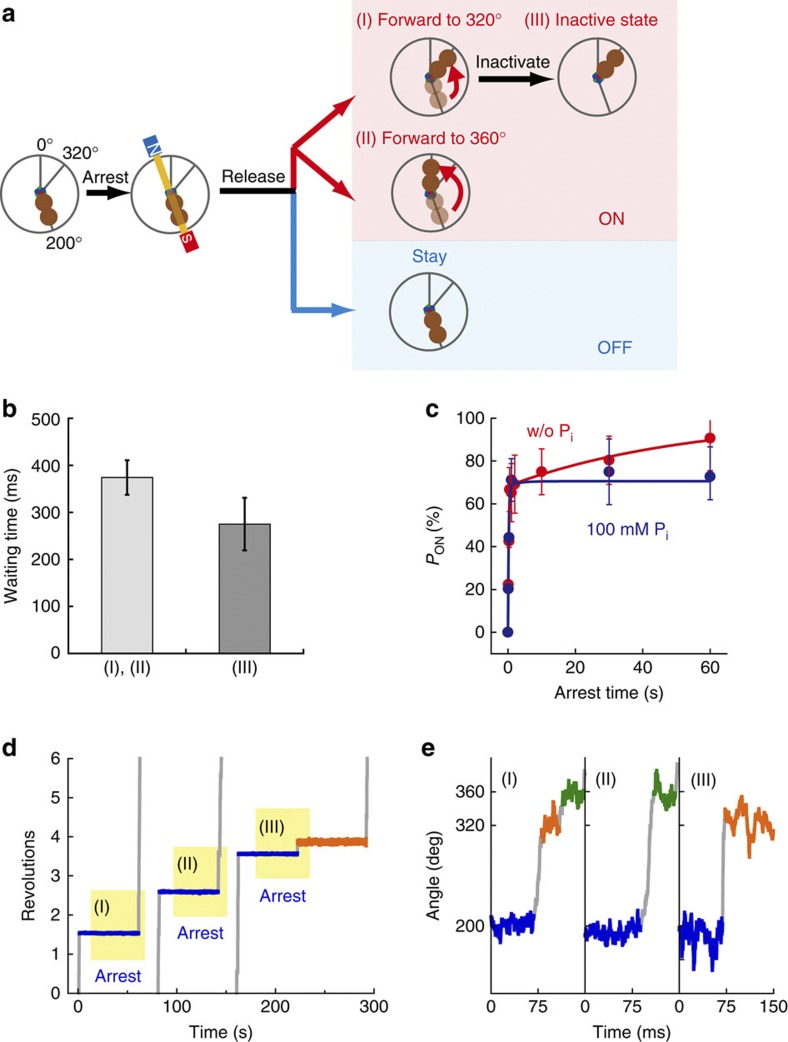
Figure 2. Single-molecule manipulation for Pi release.
(a) Schematic representation of manipulation procedures. When the hybrid F1-ATPase paused at 200°; that is, the angle for ATP hydrolysis by β(E190D), we switched on the magnetic tweezers and arrested F1 at ±10° from 200°. After release from arrest, F1-ATPase roughly showed two behaviours: rotating forward to next reaction angle (‘ON’), or staying at the original pausing angle (‘OFF’). In addition, the behaviour of ‘ON’ was classified into three types: (I) rotating forward to 320° and showing the distinctive pause for Pi release (time constant (τ) is ~9 ms); (II) rotating forward to 360° (skip the pause at 320°); or (III) rotating forward to 320° and showing a long pause (τ=~39 s). (b) Hydrolysis-waiting time at 200° before F1-ATPase is arrested with the magnetic tweezers. The average waiting time was 370±37 ms for the behaviours (I) and (II) (light grey), and 275±56 ms for the behaviour (III) (grey). Error bars indicate s.d. (c) Time course of PON. Red and blue points represent PON in the absence or presence of 100 mM Pi, respectively. The number of arrest trials and molecules used for each data point were 23–108 and 8–19. The time courses were fitted with the reaction scheme; F1˙ATP↔˙F1 (ADP+Pi)→F1˙ADP+Pi (solid lines). (d) Example of the time course of the arrest experiments (blue periods) corresponding to the behaviour of (I), (II) and (III), respectively. (e) Magnification of d. Orange and green represent the pauses at 320° and 360°.