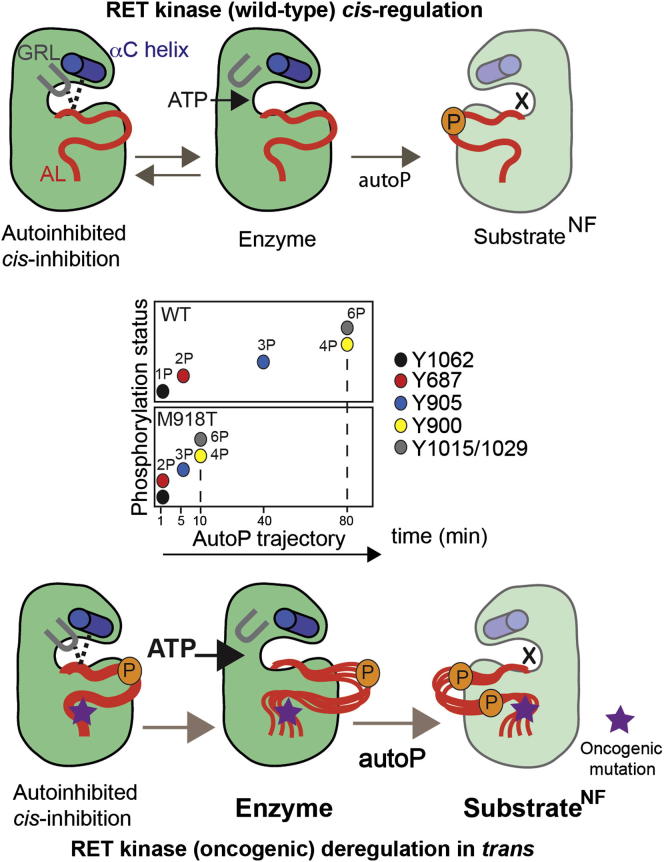
Figure 7.
A Proposed Model for RET (WT) Kinase cis-Regulation and Oncogenic Perturbation of the AutoP Trajectory In trans
Inset: summary of changes to the autoP trajectory by oncogenic RET M918T (quantified by LFQMS based on saturation rates). This is caused by an enhanced enzymatic activity and higher affinity for ATP and the production of a better intermolecular RET substrate. RET WT KD cis-regulation by a tether affecting GRL and AL conformation leading to autoinhibition. An ATP-bound conformer disrupts the GRL tether between E734 (GRL), R912 (AL), and D771 (αC helix) allowing ATP to access the active site (enzyme, active kinase) and releasing the AL. This leads to phosphorylation in trans (autoP) of a RET kinase presented as a substrate. Oncogenic RET M918T kinase is autoinhibited in cis by the GRL closed conformer despite displaying enhanced phosphorylation status. Enhanced ATP affinity favors an open GRL conformer and a more active enzyme, which also displays enhanced substrate presentation by promoting more flexible “AL-out” conformers (X denotes in a nucleotide-free state of a catalytically impaired RET kinase presented as a surrogate substrate for autophosphorylation in trans).