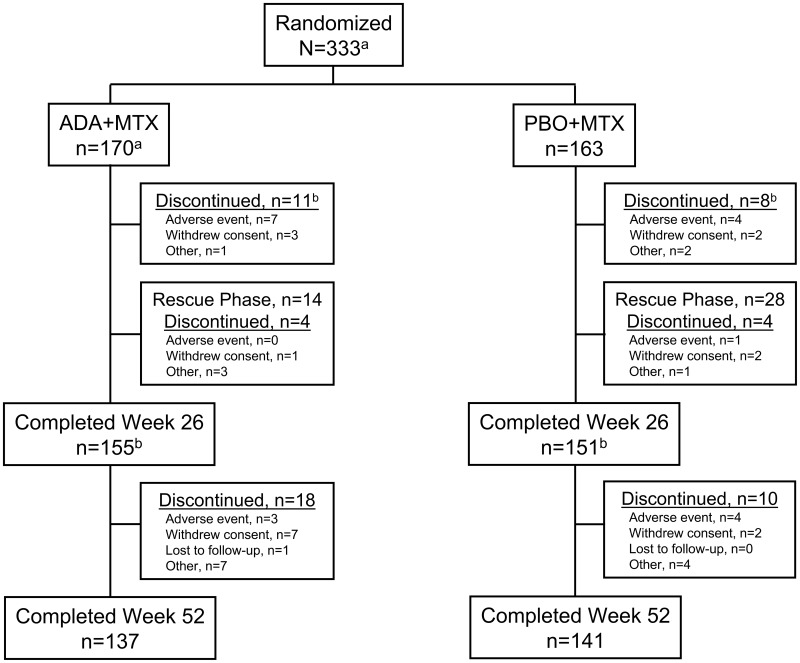
Fig. 1.
Patient disposition through week 52.
aPPS. One patient randomized to ADA + MTX received two doses of study drug at baseline and was excluded from this analysis. bThree patients in the ADA + MTX group and one in the PBO + MTX group discontinued from the study at week 26. ADA: adalimumab; PBO: placebo.