Figure 2.
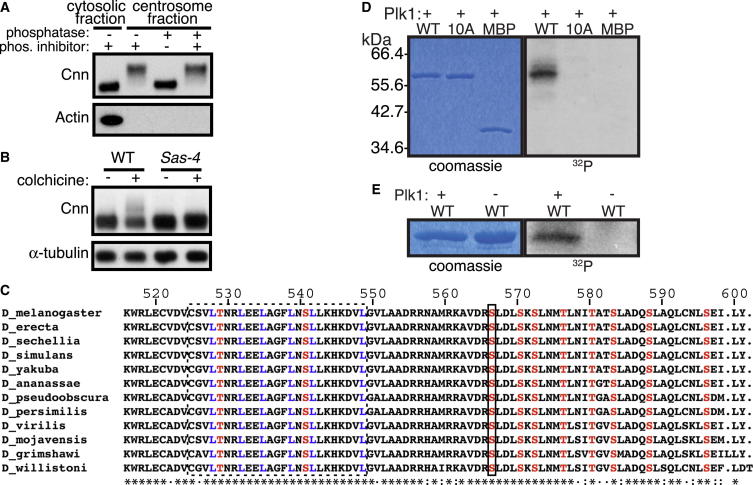
Polo/Plk1 Appears to Phosphorylate Cnn Specifically at Centrosomes
(A) Western blot of cytosolic (lane 1) and centrosomal (lanes 2–4) fractions of embryo extracts probed for Cnn (top panel) and Actin (bottom panel). Treatment of the extracts with (+) or without (−) either alkaline phosphatase (phosphatase) or a cocktail of phosphatase inhibitors (phos. inhibitor) is indicated. Cnn displays a mobility shift in the centrosome fraction (lane 2), which is abolished after phosphatase treatment (lane 3), but not if phosphatase inhibitors are included (lane 4).
(B) Western blot of interphase (−colchicine) or mitotic (+colchicine) extracts of larval brains from wild-type (WT, lanes 1 and 2) or Sas-4 mutants (lanes 3 and 4); α-tubulin is shown as a loading control. Some of the Cnn displays a mobility shift in WT mitotic extracts (lane 2) that is not seen in Sas-4 mutant extracts (lane 4), indicating that the shift is dependent on centrosomes.
(C) Alignment of the Cnn PReM domain from D. melanogaster (K516-Y601) and various other Drosophila species. This domain contains a predicted leucine zipper (dotted line box, Leu residues in blue) and ten conserved Ser/Thr residues (in red); the black box indicates S567, identified as a phosphorylation site by MS. Residue numbers for D. melanogaster are indicated.
(D and E) Coomassie-stained gels (left) and autoradiograms (right) from an in vitro kinase assay with (+) or without (−) recombinant human Plk1, containing either WT MBP-Cnn462-608 (WT) or mutant MBP-Cnn462-608 in which all ten conserved Ser/Thr residues have been mutated to Ala (10A). Only the WT protein is phosphorylated by Plk1; note that phosphorylation leads to only a very small mobility shift in these fragments.
See also Table S1.