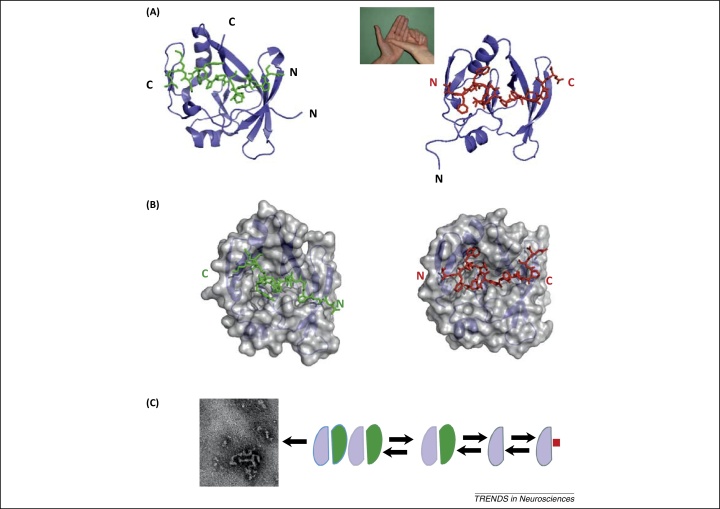
Figure 4.
Interaction with the protein CIC suggests a possible approach to drug design. (A) Comparison of the structures of the AXH domain in the dimer (left panel, PDB accession code 1oa8, [21]) and of a complex with a synthetic peptide spanning the sequence of the CIC N terminus (right panel, PDB accession code 2m41[49]). The structure of the complex corresponds, in our analogy, to having the palm wide open because the N terminus is now pushed out. (B) The same as in (A) but representing the AXH monomers with the Van der Waals surface. The N terminus of the other protomer in the dimeric form (in green) packs in the same groove occupied by the CIC peptide (in red). The two interacting chains adopt opposite orientations. [Note that the two structures were independently solved in the crystal (the dimer) and in solution (the complex). This explains the different looking van der Waals envelope that is determined by the side chain rotamers.] (C) A schematic model of the equilibrium between multiple species of the AXH domain in solution and how this can relate to aggregation. The presence of CIC (shown as a red square) shifts the equilibrium towards the monomeric form thus stabilizing the protein against aggregation. Abbreviations: CIC, Capicua; PDB, Protein Data Bank.