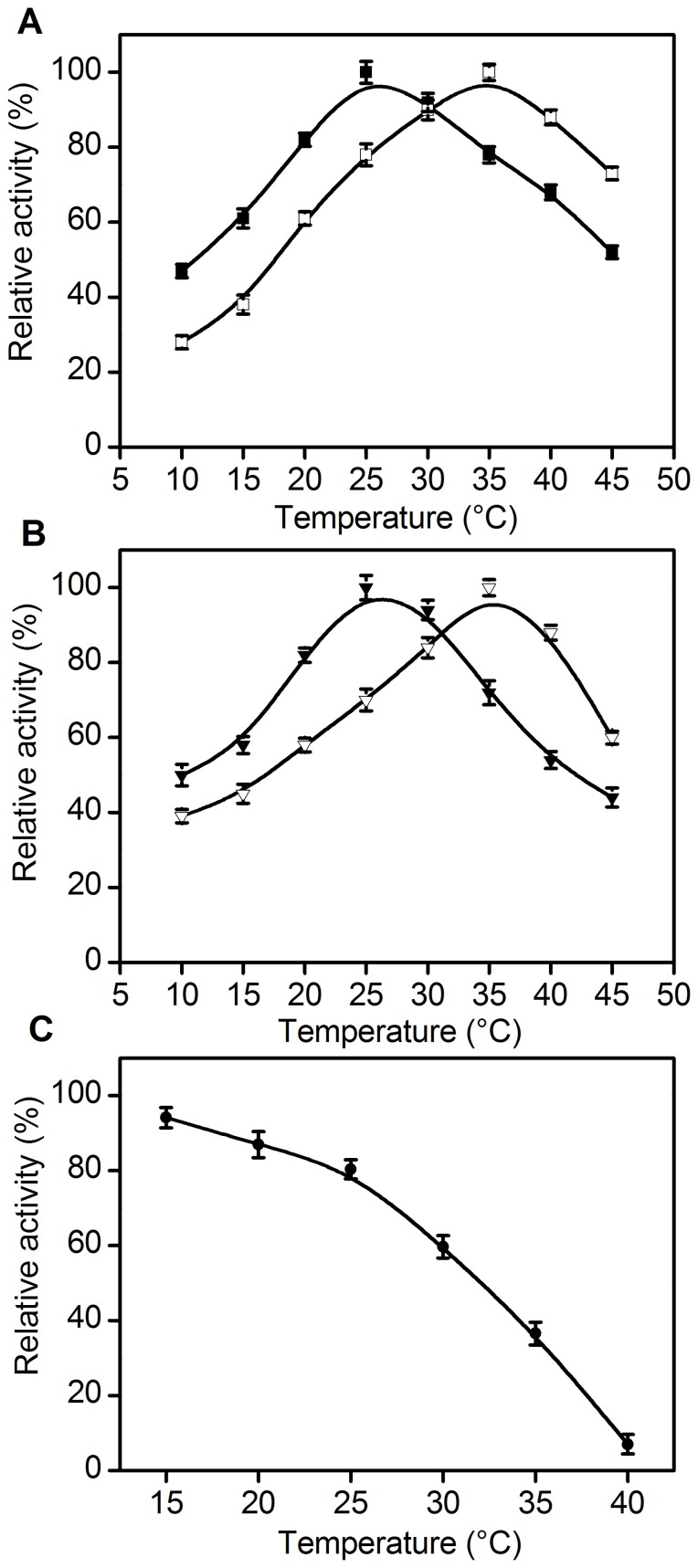
Figure 3. Relative activity of AcCR at different temperatures.
(A) Reduction of 4′-chloroacetophenone using NADPH as cofactor (▪); Oxidation of isopropanol using NADP+ as cofactor (□); (B) Reduction of 4′-chloroacetophenone using NADH as cofactor (▾); Oxidation of isopropanol using NAD+ as cofactor (□); The activity was measured in the temperature range of 10–45°C. The pH value was 5.0 (NADH as cofactor) or 7.5 (NADPH as cofactor) for reduction of 4′-chloroacetophenone or 8.0 for oxidation of isopropanol, respectively. The relative activity of AcCR at each optimal temperature for reduction and oxidation reactions was defined as 100%. (C) Thermal stability (•). The residual activity of AcCR after 5 h incubation (buffer pH 6.5) at varying temperatures were measured at 25°C.