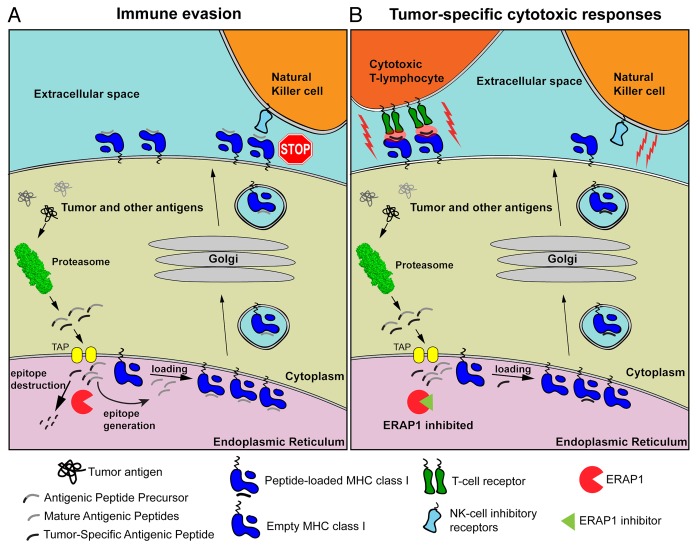
Figure 1. Mechanisms by which the inhibition of ERAP might activate cell-mediated antitumor immune responses. (A) Tumor-associated as well as normal intracellular proteins are catabolized by the proteasome, generating mature antigenic peptides as well as several antigenic peptide precursors. In the endoplasmic reticulum (ER) lumen, ER aminopeptidase 1 (ERAP1) can destroy some antigenic epitopes and at the same time process peptide precursors to generate mature antigenic epitopes for presentation by MHC class I molecules on the cell surface. Since some-tumor associated antigens are also destroyed by ERAP1, tumor-specific cytotoxic T lymphocyte (CTL) responses are evaded. In addition, peptide-loaded MHC class I molecules inhibit the cytotoxic activity of natural killer (NK) cells. (B) In the presence of ERAP1 inhibitors, tumor-associated antigens are spared from degradation and rather loaded onto MHC class I molecules. These complexes are normally translocated on the cell surface, where they can be recognized by CTLs and hence drive cytotoxic antitumor responses. Alternatively, the decline in the abundance of mature antigenic peptides due to reduced ERAP1 activity can inhibit the translocation of MHC class I molecules on the cell surface, or result to empty or misfolded MHC class I molecules that are unable to engage the inhibitory NK-cell receptors (i.e., Ly49), hence promoting NK cell-mediated cytotoxic responses.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.