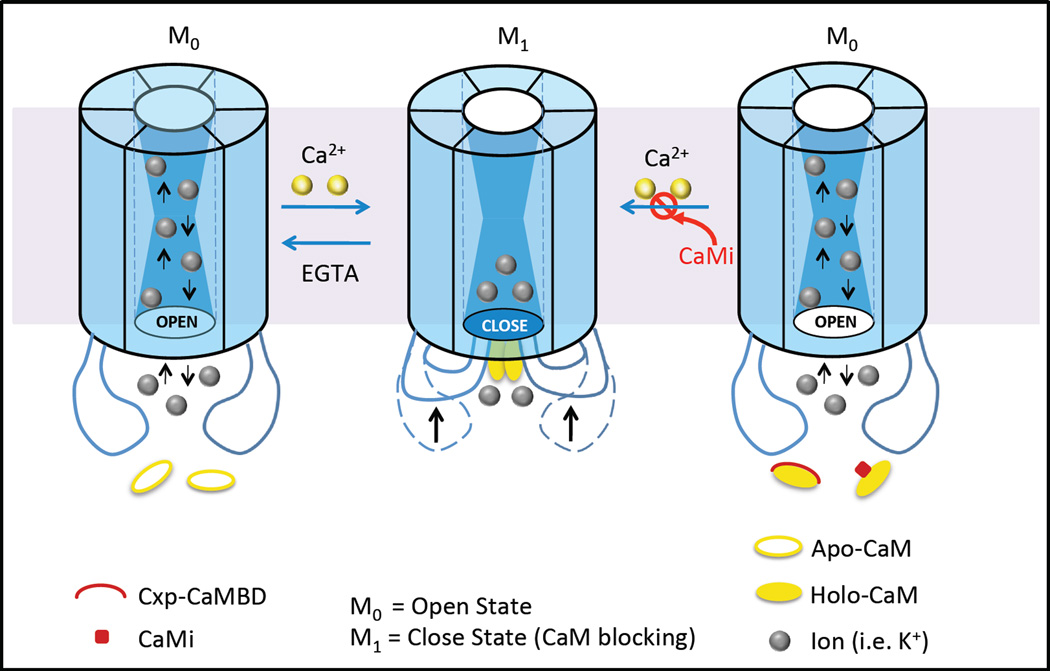
Figure 4. A proposed gating model for the mechanism of the closure of Cx43 gap junction channels by direct Ca2+/CaM binding.
In this model, connexin has one open (Mo) state and open closed state (M1). The induction of a conformational change in the cytoplasmic loop (CL) of Cx43 through Ca2+/CaM binding, steric hindrance of CaM binding near the cytoplasmic opening of the transmembrane pore, or a combination of both steric hindrance and conformational gating mechanisms causes the closure of the channel. Addition of the Cx43-CL-derived peptides or a CaMi (CaM inhibitor e.g., CDZ), prevents the Ca2+/CaM-Cx43 cytoplasmic loop interaction which changes the status of the channel from closed (M1) to the open (M0) state.