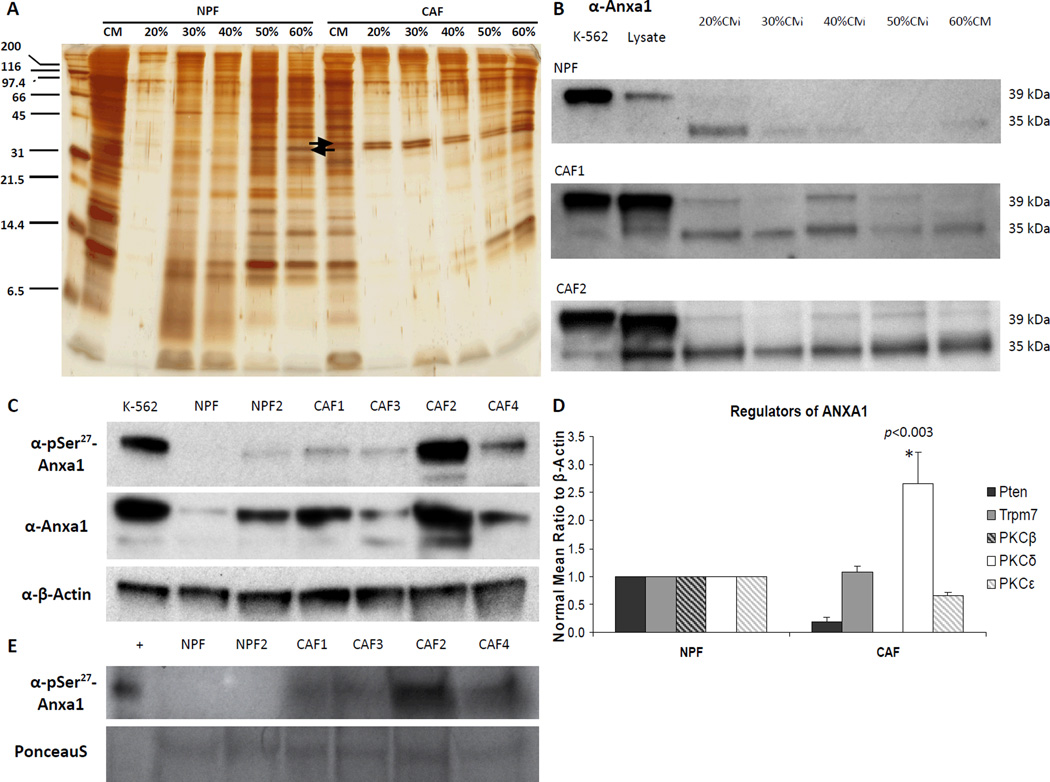
Figure 2.
AnxA1 is secreted from CAF. A, AS precipitated NPF and CAF CM protein fractions were run on an electrophoresis gel for silver stain. A pronounced band was found to be present in the CAF CM and CAF CM AS fractions. This band was weak or lacking in the NPF CM or NPF CM AS fractions. CAF CM AS bands were removed and analyzed using mass spectrometry (MS). AnxA1 was implicated from the results of MS. B, Western blot using antibody against AnxA1 confirmed the presence of AnxA1 in stromal cultures. K-562 positive control lysate was used as a loading control for exposure time between western blots of CM fractions. α-AnxA1 stained positive for two bands in blots in NPF CM fractions, CAF and CAF CM fractions. Both bands were more robust in CAF and CAF CM fractions compared to NPF, and the upper band that was more prevalent in CAF CM fractions was correlated with biological activity (Supplementary Fig. S2). Highest abundance of AnxA1 was found in the 30–40% AS cut of CAF CM. C, Phospho-serine specific antibody recognizing the amino acid motif ± 3 amino acids surrounding p-Ser27 against NPF and CAF lysates revealed more p-Ser27–AnxA1 in CAF upper band. D, Serine phosphorylator, PKCδ is upregulated in CAF. K-562 is positive control for AnxA1. *, P < 0.01. E, Phospho-serine specific antibody recognizing the amino acid motif ± 3 amino acids surrounding p-Ser27 against NPF and CAF CM revealed more p-Ser27–AnxA1 present in CM from CAFs than NPFs. CAF2 lysate was used as a positive control. Equal loading was assessed by Ponceau S solution stain.