We read with interest the recent manuscript by Bluteau et al. [1], which enhances our understanding of the molecular mechanisms underlying the changes in megakaryopoiesis that occur during human development. Specifically, the authors analyzed the transcriptome of megakaryocytes (MKs) derived from human progenitors (CD34+ cells) obtained at different stages of development: embryonic (MKES), fetal liver (MKFL), cord blood (MKCB), and adult peripheral blood (MKAD). This approach revealed a hierarchical clustering in the gene expression profiling according to MK ontogeny. During development, genes and transcriptional factors involved in cell cycle progression, polyploidization, MK development and platelet production were enriched (MKES<MKFL<MKCB<MKAD). This suggested that, during ontogeny, MKs undergo a progressive process of specification aimed at producing functional platelets [2].
Next, these investigators evaluated whether the observed developmental differences in gene expression could be regulated at the post-transcriptional level by microRNAs (miRNAs). These are small non-coding RNA molecules that modulate the expression of most protein-coding genes by either degrading target mRNAs or repressing mRNA translation [3]. To investigate this, they used a microRNA microarray and then looked for inverse correlations between the expression levels of miRNAs and their predicted target genes [1].
CXCR4 is one of the genes Bluteau et al. found to be up-regulated during ontogeny, with CXCR4 mRNA expression progressively increasing from very low levels in MKESs and yolk sac-derived MKs to intermediate levels in MKCBs and highest levels in MKADs. CXCR4 is the main receptor for Stromal Cell Derived Factor-1 (SDF-1), and important chemokine and Thrombopoietin (Tpo)-independent thrombopoietic factor [4]. In several computational databases, CXCR4 is a predicted target of miR-9 and miR-224, two miRNAs that were expressed at higher levels in MKESs than in MKADs in the Bluteau´s microarray [1]. Based on this inverse correlation, the authors suggested that these miRNAs could regulate the developmental differences in the CXCR4 expression in human MKs.
Consistent with these observations, our group found in a microRNA PCR array (MAH-103A, SABiosciences) that human miR-9 (hsa-miR-9) expression levels were 20-fold higher in CB-derived compared to adult peripheral blood (PB)-derived MKs [5, 6]. In the present study, we use real time RT-PCR and Western Blot to confirm that human neonatal MKs have significantly lower CXCR-4 mRNA and protein levels than adult MKs, and demonstrate that miR-9 regulates CXCR-4 in a megakaryocytic cell line.
Briefly, human CD34+ cells were isolated from term CB samples, and mobilized adult PB CD34+ cells were purchased from the Fred Hutchinson Cancer Institute. MKs were generated by culturing CD34+ cells in serum-free medium with rTpo (50ng/mL), as previously described [7]. The human megakaryoblastic leukemia cell line Meg-01 was maintained in DMEM+10% FBS. Expression levels of hsa-miR-9 and CXCR-4 mRNA were determined by quantitative RT-PCR and were normalized against U6 (Cat# MPH01653A, SABiosciences) and beta-actin, respectively. Primers were as follow: for CXCR-4: forward-tcg tgc caa agc ttg tcc ctg- and reverse-gcg gta acc aat tcg cga ata gtg c-; For actin: forward -gag cgg gaa atc gtg cgt gac a- and reverse -agg aag gaa ggc tgg aag agt gc-; for hsa-miR-9: forward –gccgtgtctttggttatctagc-; and reverse - tggttccaacccgactatctca-. CXCR-4 protein levels were determined by Western Blot using a rabbit anti-CXCR-4 (Abcam 2074) and a rabbit anti-beta-actin (Sigma) antibodies. CXCR-4 protein levels were quantified by densitometry using the ImageJ system, and expressed as ratio relative to beta-actin. Meg-01 cells were nucleofected with pre-miR- 9 or control oligonucleotides (Ambion), using the Basic Nucleofector Kit (Amaxa Inc). Cells were harvested 24 and 72 hrs after nucleofection for miR-9 and CXCR-4 protein expression studies, respectively.
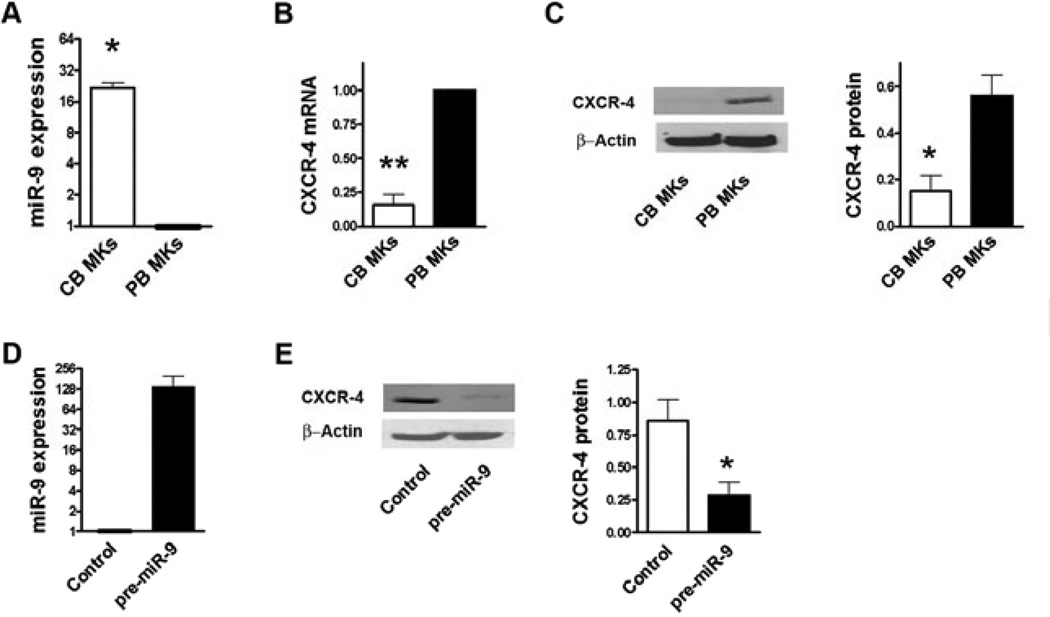
As a first step, we evaluated CXCR-4 expression in human MKs. As predicted from their differences in miR-9 expression (Fig. 1A), and consistent with Bluteau et al., CXCR-4 m-RNA levels were lower in CB-derived compared to adult PB-derived MKs (p<0.01; Fig 1B). Next, we measured CXCR-4 protein expression in CB- vs PB-derived MKs, and confirmed that the developmental differences in CXCR-4 mRNA expression were also evident at the protein level (Fig. 1C). Rivière and collaborators also reported lower flow cytometric expression of CXCR-4 in CB-derived MKs (range 30-100%) compared to adult bone marrow-derived MKs (range 72% to 100%) [8]. These findings are also consistent with those of Mazharian et al. in murine MKs [9].
Figure 1. hsa-miR-9 regulates the expression of CXCR-4.
CXCR-4 and hsa-miR-9 levels were measured in human primary MKs derived from cord blood (CB) and from adult peripheral-blood (PB) CD34+ progenitors. (A) hsa-miR-9 values were normalized against U6 (internal control), and CB values were expressed as a ratio to adult PB values. hsa-miR-9 levels were significantly higher in CB-derived compared to adult PB-derived mature MKs. (B) CXCR-4 mRNA levels, expressed as a ratio to beta actin, were significantly lower in CB-derived compared to adult PB-derived MKs. (C) CXCR- 4 protein levels (ratio to beta actin) were significantly lower in CB-derived compared to adult PB-derived MKs. (D) To determine whether CXCR-4 is regulated by miR-9 in MKs, Meg-01 cells were nucleofected with either pre-miR-9 or a non-targeting scrambled oligonucleotide as control. hsa-miR-9 expression was 136.2±63.9 fold higher in cells nucleofected with pre-mir-9 than in cells nucleofected with the control oligo. (E) CXCR-4 protein expression was consistently down-modulated in cells over-expressing miR-9, as shown in this representative Western Blot and the bar graph summarizing the results of multiple experiments. Bar graphs represent the mean±SEM of at least 3 independent experiments. Mann-Whitney tests were used to compare the two groups. Statistical difference was defined as p<0.05 using SSPS15.0. *P<0.05. **P<0.01.
To investigate whether miR-9 regulates CXCR-4 protein expression, we then transfected Meg-01 cells with either pre-miR-9 or a non-targeting scrambled oligonucleotide (control). Cells transfected with pre-miR-9 had a >100-fold increase in miR-9 levels (Fig 1D), and exhibited significantly lower CXCR-4 protein levels (p=0.02; Fig 1E).
Over the last decade, multiple reports have unveiled critical roles of miRNAs in megakaryopoiesis [10]. Labbaye et al. reported that the downregulation of CXCR4 by overexpression of miR-146a impaired the MK differentiation/maturation of human CD34+ cells [11], although this observation was not confirmed in later studies [12]. Additionally, disorders of miRNA expression have been associated with hematological diseases. It has been postulated that miR-9 contributes to the phenotype of Hodgkin/Reed-Sternberg cells by interfering with normal B-cell terminal differentiation [13]. MiR-9 is also downregulated in many type of cancers, and it has been suggested that it functions as a tumor suppressive miRNA by repressing CXCR4 expression [14].
SDF-1 is a CXC chemokine whose receptor, CXCR-4, is expressed on hematopoietic stem cell, osteoblasts, MKs, T cells and other inflammatory cells. SDF-1 has been shown to potently stimulate the maturation of human PB-derived MKs in vitro [15], and of murine MKs in vivo [16]. In vivo, the maturational effects of the SDF-1/CXCR-4 axis are thought to be mediated by stimulating the migration of MKs from the bone marrow endosteal to the endothelial niche. Importantly, the administration of SDF-1 in conjunction with fibroblast growth factor-4 to Tpo−/− or c-mpl−/− mice restored their platelet counts to wild type levels, demonstrating the Tpo-independent nature of this pathway in vivo [16].
In recent years, we and other groups have shown substantial cellular and molecular differences between fetal/neonatal and adult megakaryocytes [7] involving transcription factors, signaling pathways [17, 18] and miRNAs [5]. Our current findings, combined with those of Bluteau et al., support that CXCR-4 expression is down-regulated by miR-9 in human fetal and neonatal MKs. Given the importance of the SDF-1/CXCR-4 axis in MK migration and as a Tpo-independent thrombopoietic pathway, these developmental differences might underlie the delayed platelet engraftment following transplantation with CB stem cells, and perhaps some of the manifestations of congenital amegakaryocytic thrombocytopenia in neonates. A better understanding of the molecular basis for the unique phenotype of fetal and neonatal MKs is critical to elucidate the pathogenesis of these and other MK disorder that exclusively or predominantly affect neonates and infants, including thrombocytopenia-absent radius, the transient myeloproliferative disorder associated with trisomy 21 and GATA-1s mutations, and Jacobsen syndrome.
Acknowledgements
This work was supported by NIH grant HL69990 (MSV) and Grant BAE-90058 from the Health Research Fund, Ministry of Health, Spain (FFM).
Footnotes
Authorship Contributions
F. Ferrer-Marin, R. Gutti and Z. J. Liu designed and performed experiments, collected and analyzed data, and wrote the manuscript; M. Sola-Visner supervised and designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Bluteau O, Langlois T, Rivera-Munoz P, Favale F, Rameau P, Meurice G, Dessen P, Solary E, Raslova H, Mercher T, Debili N, Vainchenker W. Developmental changes in human megakaryopoiesis. J Thromb Haemost. 2013;11:1730–1741. doi: 10.1111/jth.12326. [DOI] [PubMed] [Google Scholar]
- 2.Ghevaert C. Megakaryopoiesis through the ages: from the twinkle in the eye to the fully grown adult. J Thromb Haemost. 2013;11:1727–1729. doi: 10.1111/jth.12349. [DOI] [PubMed] [Google Scholar]
- 3.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 4.Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009:147–152. doi: 10.1182/asheducation-2009.1.147. [DOI] [PubMed] [Google Scholar]
- 5.Gutti R, Sallmon H, Bailey M, Liu ZJ, Sola-Visner M. MicroRNA expression signatures are different in human neonatal compared to adult megakaryocytes. J Thromb Hemost. 2009;7(S2):955. [Google Scholar]
- 6.Ferrer-Marin F, Gutti R, Liu ZJ, Italiano JE, Jr, Hu Z, Slayton W, Bailey M, Sola-Visner M. Neonatal Megakaryocytes Do Not Mature Normally In the Absence of Thrombopoietin In Vivo: Potential Role of Developmental Differences In CXCR4 Expression. Blood (ASH Annual Meeting Abstracts) 2010;116:88. [Google Scholar]
- 7.Liu ZJ, Italiano J, Jr, Ferrer-Marin F, Gutti R, Bailey M, Poterjoy B, Rimsza L, Sola-Visner M. Developmental differences in megakaryocytopoiesis are associated with up-regulated TPO signaling through mTOR and elevated GATA-1 levels in neonatal megakaryocytes. Blood. 2011;117:4106–4117. doi: 10.1182/blood-2010-07-293092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riviere C, Subra F, Cohen-Solal K, Cordette-Lagarde V, Letestu R, Auclair C, Vainchenker W, Louache F. Phenotypic and functional evidence for the expression of CXCR4 receptor during megakaryocytopoiesis. Blood. 1999;93:1511–1523. [PubMed] [Google Scholar]
- 9.Mazharian A, Watson SP, Severin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37:1238–1249. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein LC, McKenzie SE, Shaw C, Holinstat MA, Kunapuli SP, Bray PF. MicroRNAs in platelet production and activation. J Thromb Haemost. 2013;11(Suppl 1):340–350. doi: 10.1111/jth.12214. [DOI] [PubMed] [Google Scholar]
- 11.Labbaye C, Spinello I, Quaranta MT, Pelosi E, Pasquini L, Petrucci E, Biffoni M, Nuzzolo ER, Billi M, Foa R, Brunetti E, Grignani F, Testa U, Peschle C. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nat Cell Biol. 2008;10:788–801. doi: 10.1038/ncb1741. [DOI] [PubMed] [Google Scholar]
- 12.Opalinska JB, Bersenev A, Zhang Z, Schmaier AA, Choi J, Yao Y, D'Souza J, Tong W, Weiss MJ. MicroRNA expression in maturing murine megakaryocytes. Blood. 2010;116:e128–e138. doi: 10.1182/blood-2010-06-292920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie K, Gomez M, Landgraf P, Garcia JF, Liu Y, Tan LH, Chadburn A, Tuschl T, Knowles DM, Tam W. MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in Hodgkin lymphomas. Am J Pathol. 2008;173:242–252. doi: 10.2353/ajpath.2008.080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J, Luo H, Liu X, Peng Y, Zhang B, Wang L, Xu X, Peng X, Li G, Tian W, He M, Hsiangfu K, Li X. miR-9 targets CXCR4 and functions as a potential tumor suppressor in nasopharyngeal carcinoma. Carcinogenesis. 2013 doi: 10.1093/carcin/bgt354. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Guerriero R, Mattia G, Testa U, Chelucci C, Macioce G, Casella I, Samoggia P, Peschle C, Hassan HJ. Stromal cell-derived factor 1alpha increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood. 2001;97:2587–2595. doi: 10.1182/blood.v97.9.2587. [DOI] [PubMed] [Google Scholar]
- 16.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZJ, Sola-Visner M. Neonatal and adult megakaryopoiesis. Curr Opin Hematol. 2011;18:330–337. doi: 10.1097/MOH.0b013e3283497ed5. [DOI] [PubMed] [Google Scholar]
- 18.Klusmann JH, Godinho FJ, Heitmann K, Maroz A, Koch ML, Reinhardt D, Orkin SH, Li Z. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev. 2010;24:1659–1672. doi: 10.1101/gad.1903410. [DOI] [PMC free article] [PubMed] [Google Scholar]