Figure 3.
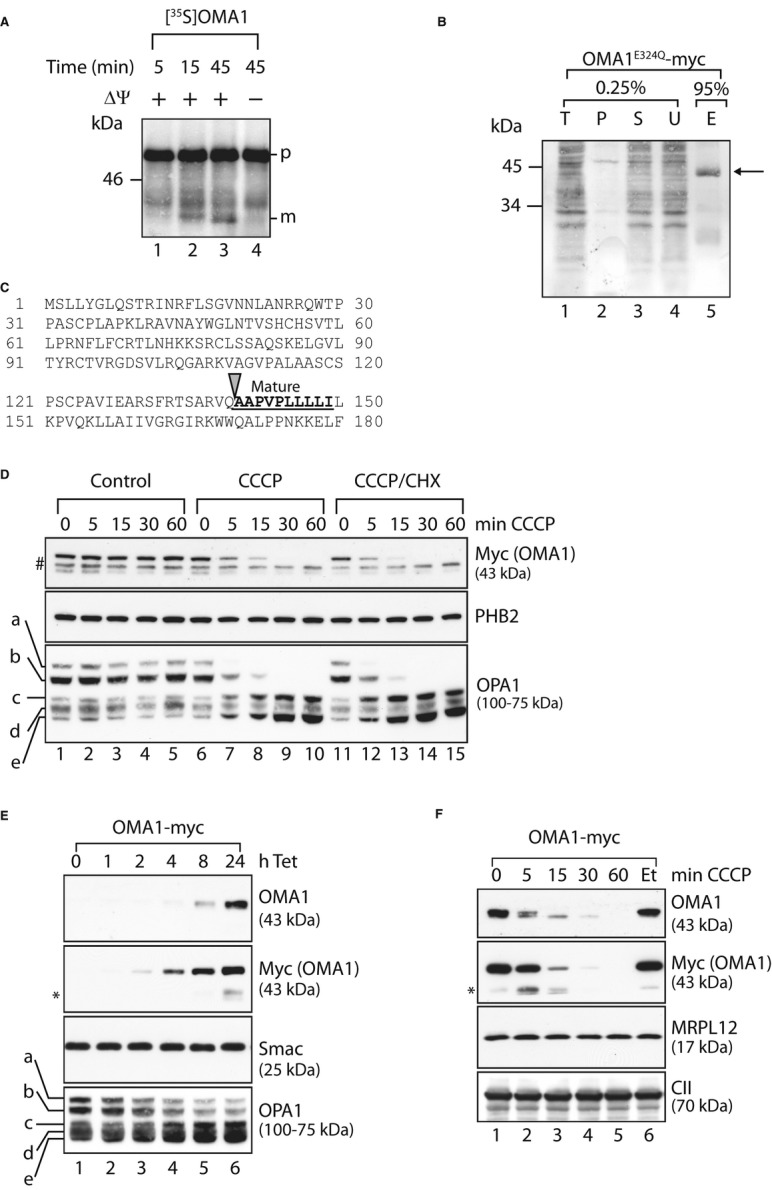
- In vitro import of OMA1 into mitochondria. Mitochondria were isolated from MEFs and incubated with [35S]-labelled OMA1 that was synthesised in a cell-free system for the time points indicated in the presence or absence of membrane potential (ΔΨ). Mitochondrial proteins were analysed by SDS–PAGE and autoradiography. p, precursor form; m, mature form.
- N-terminal sequencing of mature OMA1. Mitochondria isolated from HEK293 cells expressing OMA1E324Q-myc were solubilised in 1% (v/v) NP-40 and subjected to immunoprecipitation using myc-specific antibodies. Total (T), pellet (P), supernatant (S) and unbound (U) fractions (0.25%) and the eluate (E; 95%) were analysed by SDS–PAGE and Coomassie staining. The arrow indicates OMA1E324Q-myc in the eluate.
- N-terminal amino acid sequence of OMA1. Mature OMA1 is generated upon proteolytic cleavage at the site indicated with an arrow. The bold, underlined sequence represents the N-terminal sequence of mature OMA1 determined by Edman degradation.
- Degradation of pre-existing OMA1 following mitochondrial stress. MEFs stably expressing OMA1-myc were treated with CCCP, with cyclohexamide (CHX) or with ethanol and DMSO (control) as indicated. Samples were isolated and analysed via SDS–PAGE and immunoblotting. #, non-specific cross reaction; a-e, OPA1 forms.
- Overexpression of OMA1-myc in HEK293 cells. OMA1-myc expression was induced by addition of tetracycline (Tet) for the time points shown. Cell extracts were analysed by SDS–PAGE and immunoblotting. *, proteolytic fragments of OMA1.
- Overexpressed OMA1 is degraded following mitochondrial dysfunction. After tetracycline-induced expression of OMA1-myc, HEK293 cells were treated with CCCP or, for control, ethanol (Et) as indicated. Samples were assessed by SDS–PAGE and immunoblotting. *, C-terminal, proteolytic fragments of OMA1.
Source data are available online for this figure.
