Figure 2.
The C-helix is critical for mitochondrial calcium uptake.
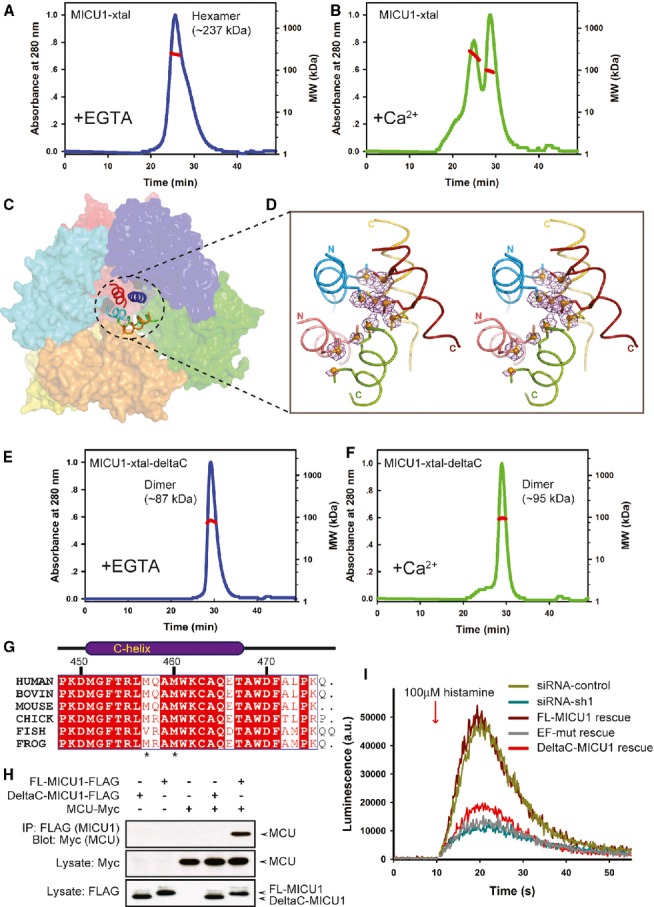
A, B Multi-angle laser scattering analysis of MICU1-xtal in the absence or presence of Ca2+. MALS-determined molecular weights were calculated over prominent peaks (red line).
C The hexameric packing of Ca2+-free MICU1. The surface map of six molecules in the asymmetric unit is shown. A cartoon model of the C-helix is presented in the center of the hexamer.
D The anomalous scattering map of the selenium-substituted Ca2+-free MICU1-xtal structure. The map colored in magenta was contoured at 3.0σ. Cartoon representations of the five C-helices from different Ca2+-free MICU1 molecules are shown. One C-helix is not included in the final model due to poor electron density. The methionines are depicted as sticks, and Se atoms are depicted as spheres.
E, F Multi-angle laser scattering analysis of MICU1-xtal-deltaC in the absence or presence of Ca2+.
G Sequence alignment of the MICU1 protein from human (Q9BPX6), bovine (Q0IIL1), mouse (Q8VCX5), chick (E1BWC6), fish (A4IG32), and frog (B1H2N3). The residues that are conserved among the species are highlighted in red. The residue numbers of human MICU1 are shown in black.
H Western blot analysis of co-immunoprecipitated wild-type FL-MICU1 and mutant DeltaC-MICU1 with the MCU channel in the absence of Ca2+.
I cDNA rescue of the RNAi mitochondrial calcium uptake phenotype.
Source data are available online for this figure.
