Figure 1.
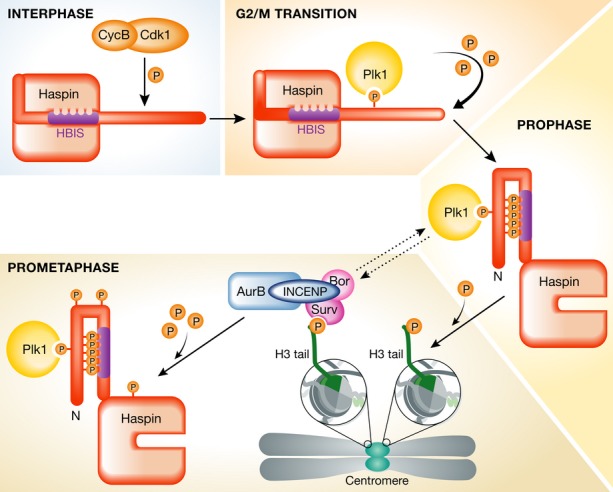
Cell cycle regulation of Haspin, the H3T3 kinase.
Haspin is an atypical kinase that is kept inactive during interphase through the interaction of a specific region in its N-terminus—enriched in basic amino acids and known as Haspin basic inhibitory segment (HBIS)—with its kinase domain. Upon mitotic entry, the Cdk1/cyclin B complex phosphorylates a specific threonine residue (T128) on human Haspin (T206 in Xenopus), generating a polo-box domain recognition site on Haspin. During prophase, Plk1 heavily phosphorylates the N-terminus of Haspin, leading to its activation and resulting in the phosphorylation of the threonine 3 on histone H3 tail on threonine 3 (H3T3). This specific histone post-translation modification is necessary for the recruitment of the chromosomal passenger complex (CPC) to centromeres. During prometaphase, Haspin is also phosphorylated by the catalytic subunit of CPC—the Aurora B kinase—which ensures that H3T3 phosphorylation at centromeres is maintained.
