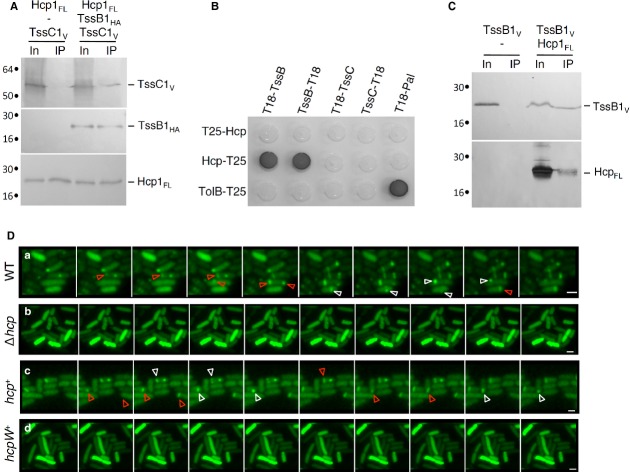
Figure 1.
Hcp1 interacts with the sheath-like TssB1 subunit and is required for sheath assembly.
A–C Interaction between T6SS tube and sheath components. Co-immunoprecipitation assay are shown in (A) and (C). Solubilized extracts of E. coli K-12 W3110 cells producing FLAG-tagged Hcp1FL (Hcp1FL), VSV-G-tagged TssC1 (TssC1V) and HA-tagged TssB1 (TssB1HA) (A) or Hcp1FL and VSV-G-tagged TssB1 (TssB1V) (C) were subjected to immunoprecipitation with anti-FLAG-coupled beads. The input (total solubilized material, In) and the immunoprecipitated material (IP) were analyzed by 12.5%-acrylamide SDS-PAGE and proteins were immunodetected with anti-FLAG, anti-VSV-G and anti-HA monoclonal antibodies. Immunodetected proteins are indicated on the right. Molecular weight markers (in kDa) are indicated on the left. Bacterial two-hybrid assay is shown in (B). BTH101 reporter cells producing the indicated fusion were spotted on LB agar plates supplemented with X-Gal. An interaction between two fusion proteins is attested by the dark color of the colony. The TolB-T25/T18-Pal combination serves as a positive control. Non-specific interactions are ruled out using TolB-T25 or T18-Pal as negative controls.
D Time-lapse fluorescence microscopy recordings of EAEC cells producing TssB1-sfGFP. Upper panel, wild-type (WT) cells; second panel, Δhcp mutant cells; third panel, Δhcp mutant cells producing Hcp1FL (hcp+); lower panel, Δhcp mutant cells producing the S158W Hcp variant (hcpW+). Red and white arrowheads point at extensions and contractions of the T6SS sheath-like structures, respectively. Each frame is separated by 30 sec. The scale bar represents 2 μm.