Figure 4.
A Minimal Consensus Sequence for PIH1 Domain Binding Reveals Potential R2TP Substrates
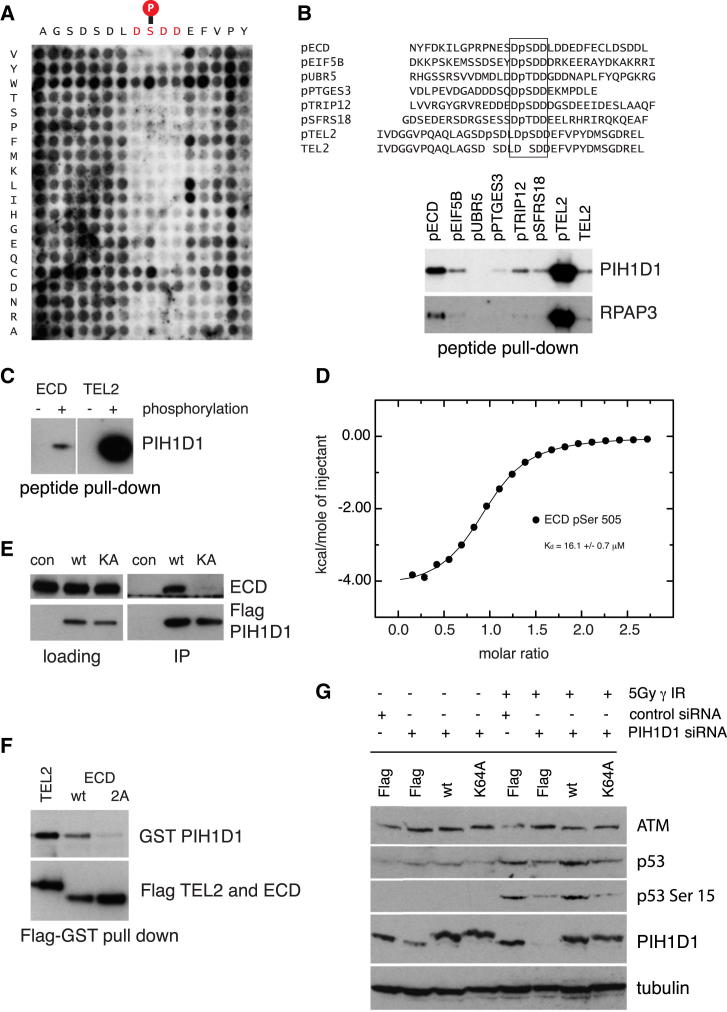
(A) A pep-spot array was synthesized in which each residue in the TEL2 phosphopolypeptide (16 amino acids) was substituted with all other amino acids. Peptides were spotted on a peptide array and incubated with purified 6× His-tagged PIH1D1 fragment 1–180.
(B) Peptide pull-down of PIH1D1 from HEK293T whole-cell extract. PIH1D1 and RPAP3 were pulled down from whole-cell extract by biotinylated peptides containing the phosphorylated consensus PIH-N domain binding sequence. Negative control (line 8) was unphosphorylated TEL2 peptide.
(C) ECD peptide binds PIH1D1 in a phosphopeptide-specific manner. PIH1D1 was pulled down from HEK293T whole-cell extract by biotinylated ECD peptide containing phosphorylated Ser505 but not with nonphosphorylated ECD peptide.
(D) ITC analysis of ECD binding (RPNESDpS505DDLDDY) to PIH1D1 1–180.
(E) FLAG-tagged WT PIH1D1, but not K64A mutant, immunoprecipitates ECD from HEK293T whole-cell extract. Proteins were immunoprecipitated from HEK293T cells transiently transfected with FLAG-tagged PIH1D1 proteins or empty vector expression FLAG.
(F) Mutation of ECD Ser505 and Ser518 to Ala disrupt binding of FLAG-tagged ECD to GST-tagged WT PIH1D1.
(G) Mutation of PIH1D1 leads to decreased levels of p53 after DNA damage. Retinal pigment epithelium cells were stably transfected with FLAG-tagged WT PIH1D1, PIH1D1 K64A, or empty vector expressing FLAG. The cells were treated with control siRNA (si con) or siRNA targeting 5′ untranslated region of PIH1D1 cDNA and irradiated with 5Gy. Samples were collected 1 hr after irradiation.