Abstract
Clinical research and practice support a multi-method approach to validating behavioral problems in children. We examined whether parent-reported symptoms of hyperactivity and inattention (using the Disruptive Behavior Disorder Rating Scale) were substantiated by objective laboratory measures [hyperactivity measured by wrist-worn actigraphy (ACT) and inattention assessed using a 20-minute continuous performance task (CPT)] in three age- and demographically-matched groups of school-age children: children with prenatal alcohol exposure (AE), non-exposed children with idiopathic ADHD (ADHD), and controls (CON). Results indicated that the clinical groups (AE, ADHD) had significantly higher parent-reported levels for both domains compared to the CON group, and did not differ from each other. On the laboratory measures, the clinical groups were more inattentive than controls on the CPT, but did not differ from each other. In contrast, the ADHD group had higher objective activity on the ACT than AE and CON, which did not differ from each other. Thus, laboratory measures differentially validated parent reports in a group-dependent manner. Actigraphy substantiated parent-reported hyperactivity for children in the ADHD group but not for children in the AE group, while the CPT validated parent-reported inattention for both clinical groups. Although the majority of children in the AE group met criteria for ADHD, objective activity levels were not different from controls, indicating that hyperactivity may be a less prominent feature in the AE group. Thus, while there is considerable overlap between the effects of prenatal alcohol exposure and ADHD, differences in behavioral profiles may be clinically useful in differential diagnosis. Further, these data indicate that objective measures should be used to validate parent reports.
Keywords: fetal alcohol spectrum disorders (FASD), fetal alcohol syndrome (FAS), prenatal alcohol exposure, attention-deficit/hyperactivity disorder (ADHD), hyperactivity, inattention
1. Introduction
Fetal alcohol spectrum disorders (FASD) affect approximately 2–5% of younger school children in the United States (May et al., 2009) and affected children are at risk for a constellation of cognitive, behavioral, and motor impairments (see, Mattson, Crocker, & Nguyen, 2011). These impairments occur across the lifespan (Spohr, Willms, & Steinhausen, 2007; Streissguth, 1992) and to varying degrees of severity (Mattson et al., 2011). Children with FASD often meet diagnostic criteria for a variety of disruptive disorders (Ware et al., 2013), including high rates of attentiondeficit/ hyperactivity disorder (ADHD; Fryer, McGee, Matt, Riley, & Mattson, 2007; Landgren, Svensson, Strömland, & Andersson Grönlund, 2010). Conversely, children with ADHD are 2.5 times more likely to be alcohol-exposed than those without ADHD (Mick, Biederman, Faraone, Sayer, & Kleinman, 2002). Shared characteristics between children with FASD and non-exposed children with ADHD (Halperin, Matier, Bedi, Sharma, & Newcorn, 1992) make it difficult to accurately differentiate these two groups (Mattson & Riley, 2011). For example, attention deficits are common among both children with FASD (Coles et al., 1997; Kooistra, Crawford, Gibbard, Kaplan, & Fan, 2011; Mattson, Calarco, & Lang, 2006) and idiopathic ADHD (Doig, McLennan, & Gibbard, 2008; Herman, Acosta, & Chang, 2008; LaDue, Streissguth, & Randels, 1992). Another core feature of ADHD, the presence of hyperactivity (Barkley & Murphy, 1998), has only been described using parent report, either anecdotally or qualitatively in children with FASD (Landesman-Dwyer, Ragozin, & Little, 1981; Shaywitz, Cohen, & Shaywitz, 1980), and has yet to be examined directly.
The identification of inattention and hyperactivity in children with ADHD or FASD relies primarily upon parent/guardian evaluations (Biederman, Faraone, Monuteaux, & Grossbard, 2004; McGrath, Handwerk, Armstrong, Lucas, & Friman, 2004), as they are easy to administer, require minimal interpretation, and provide information from those most experienced and invested in the child. While beneficial, these reports are subjective and may be influenced by motivation for receiving medication or other services. To increase accuracy, multi-method evaluation of hyperactivity and inattention has been employed to incorporate information from both parents and teachers, in addition to objective measures (McGrath et al., 2004; Sims & Lonigan, 2012).
Correlations between parent and teacher ratings of ADHD symptoms are generally lower than expected, ranging between .40 to .59 on several well-respected questionnaires for ADHD populations (Dupaul, 1991; Sprafkin, Volpe, Gadow, Nolan, & Kelly, 2002). For the Disruptive Behavior Rating Scale (Barkley & Murphy, 1998), parent and teacher ratings of ADHD symptoms of inattention and hyperactivity were not significantly correlated (Hartman, Rhee, Willcutt, & Pennington, 2007), even though parent and teacher ratings were significantly correlated on other behavioral scales including oppositional defiant disorder symptomology (Antrop, Roeyers, Oosterlaan, & Van Oost, 2002). Parents and teachers observe children in different environments, and while we would expect differences in behavioral ratings for a variety of reasons, an objective measure of hyperactivity to support these ratings would facilitate valid identification of behavioral issues.
In addition to parent questionnaires, objective child performance measures have been used to supplement clinical judgment of diagnosis and identification (Sims & Lonigan, 2012). Research studies have used a variety of performance measures to assess behavioral symptomology such as computerized continuous performance tasks (CPT) to assess inattention and portable electronic activity monitors (actigraphy) to assess hyperactivity (Sims & Lonigan, 2012). Continuous performance tasks are a wellvalidated measure of inattention in a variety of populations including ADHD (e.g., Letz, Pieper, & Morris, 1996) and FASD (e.g., Kooistra, Crawford, Gibbard, Ramage, & Kaplan, 2010). Actigraphy is an objective, non-invasive, quantitative method of measuring activity levels and has been used to confirm the presence of hyperactivity as a core deficit of ADHD (Halperin et al., 1992; Porrino et al., 1983). Early use of actigraphy demonstrated that children with ADHD are approximately 25 – 30% more active during academic classroom activities (Porrino et al., 1983) and laboratory-based attention tasks (Halperin et al., 1992) compared to control children. In the past twenty years, the combination of CPT and physical observation tools (e.g., actigraphy and infrared motion tracking systems) has allowed objective differentiation of children with ADHD from typically developing children (Teicher, Ito, Glod, & Barber, 1996; Teicher, Lowen, Polcari, Foley, & McGreenery, 2004).
While these measures have been successfully utilized to assess locomotor activity in children with ADHD, hyperactivity in FASD has yet to be assessed using objective measures, instead relying exclusively upon caregiver report and qualitative observations. Actigraphy has only been used once in the FASD population to assess sleep disturbances (Wengel, Hanlon-Dearman, & Fjeldsted, 2011), but not during waking hours. To our knowledge, the current study is the first to use actigraphy to objectively assess the presence of hyperactivity in the FASD population.
While there are parental, qualitiative, and anecdotal reports that substantiate hyperactivity and inattention as characteristics shared by both ADHD and FASD (Barkley & Murphy, 1998; Landesman-Dwyer et al., 1981; Shaywitz et al., 1980), understanding the precise nature of these impairments may help facilitate identification of children with FASD and inform the development of targeted interventions for this population. To this end, we examined: (1) whether laboratory measures of hyperactivity and inattention were consistent with primary caregiver reports for three groups of children (children with histories of heavy prenatal alcohol exposure, children with idiopathic ADHD, and controls); and (2) whether deficits noted in subjects with prenatal alcohol exposure are specific to this condition. We hypothesized that parents in both clinical groups would report hyperactivity and inattention and that objective laboratory measures would support these subjective measures.
2. Methods
2.1. General Methods
Children and their caregivers were recruited as part of an ongoing multi-site study supported by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD; see, Mattson, Foroud, et al., 2010). Recruitment for this CIFASD phase (Phase 1) occurred between 2003 and 2007 and recruitment methods for all children included word of mouth, clinician referral, and advertisements. Actigraphy was only used at one CIFASD site, and thus all subjects for this study were from the Center for Behavioral Teratology at San Diego State University.
Subjects were children (N = 82) between the ages of 7–18 years (M = 12.24, SD = 3.27). All children were tested individually by a trained examiner blind to subject group. Informed consent and assent were obtained for all subjects and the Institutional Review Board (IRB) approved all procedures. As part of the larger project, IQ scores were available from the Leiter International Performance Scale – Revised (Leiter-R, see Roid & Miller, 1997), a nonverbal test of intelligence for children between the ages of 2 and 20 years old. From the Leiter-R, a composite score of general cognitive ability (IQ) was derived to assess general intellectual functioning. Neuropsychological test results have been published elsewhere (Mattson, Roesch, et al., 2010).
2.2. Subjects
Three groups of subjects were included in this study: children with a history of prenatal alcohol exposure (AE, n = 44), non-exposed children with ADHD (ADHD, n = 16) and non-exposed children without ADHD (CON, n = 22). Given the high prevalence of ADHD in children with FASD, several recent studies have incorporated ADHD comparison groups (see, Mattson et al., 2011). To receive a diagnosis of ADHD in this study, children had to have at least two of the following indications of this disorder: (1) positive diagnosis of ADHD on the parent-reported Computerized Diagnostic Interview Schedule for Children-Fourth Edition, C-DISC-4.0 (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000); (2) clinical (T ≥ 70) score on the parent-reported Diagnostic and Statistical Manual (DSM)-Oriented ADHD scale from the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001); (3) a positive ADHD screen based on a checklist of the DSM-IV criteria for ADHD (American Psychiatric Association, 2000); or (4) ongoing use of medication prescribed for the treatment of ADHD. A multi-method approach of diagnosing ADHD is a well-accepted clinical practice both in research and in the DSM5 (American Psychiatric Association, 2013; Lahey et al., 1994).
Children in the AE group had confirmed histories of heavy prenatal alcohol exposure, defined as maternal consumption of more than 4 alcoholic drinks per occasion at least once per week or at least 14 drinks per week (on average) during gestation. Prenatal alcohol exposure was confirmed retrospectively using medical history, birth records, social services records, or maternal report, when available. However, direct maternal report was not common for children with histories of prenatal alcohol exposure, as many of these children no longer resided with their biological families. Thus, precise details about alcohol consumption (i.e., dose and timing) were often unavailable. In these cases, mothers were reported to be “alcoholic” or alcohol abusing or dependent in pregnancy. A diagnosis of FAS was determined by a member of the CIFASD Dysmorphology Core using a standardized assessment following diagnostic criteria, described elsewhere (Jones et al., 2006; Mattson, Foroud, et al., 2010). Within the AE group, 9 children (20.5%) met these research criteria for FAS. In addition, 34 children (77.3%) in the AE group met criteria for ADHD given the two method confirmatory strategy, and 2 additional children had subclinical levels of ADHD based on the C-DISC-4.0 (3–5 symptoms positively endorsed within the hyperactivity/impulsivity or inattention sections). Of the remaining subjects in the AE group, 2 had insufficient information upon which to base an ADHD diagnosis, 2 had inconclusive information, and 4 did not meet clinical or subclinical criteria for ADHD based on the multi-method approach. All 44 children in the AE group were included in analysis to preserve generalizeability and capture the heterogeneity of deficits associated with heavy prenatal alcohol exposure, however, exploratory analysis (described below) were conducted including only the 34 children in the AE group who met criteria for ADHD.
Children in the comparison groups (ADHD, CON) had no prenatal alcohol exposure or minimal exposure (i.e., no more than one drink per week on average and never more than two drinks per occasion during gestation). Children were also excluded from the ADHD and CON groups if they displayed subclinical symptoms of ADHD, defined as having more than minimal symptoms (> 3), yet not meeting the clinical threshold of greater than 5 symptoms. Children were excluded from all groups if they had suffered a significant head injury or loss of consciousness greater than 30 minutes, were not primary English speakers, were adopted from abroad after the age of 5 years (or less than two years prior to assessment), had other known causes of cognitive deficiency, or had a psychiatric or physical disability that prevented successful study completion.
2.3. Measures
Disruptive Behavior Disorder Rating Scale (DBD)
The DBD (Pelham, Gnagy, Greenslade, & Milich, 1992) is a 45-item parent-reported assessment of behavior that matches DSM criteria for clinically relevant behaviors. Primary caregivers endorse the description that best represents their child’s manifestation of a behavior (“Not at all”, “Just a little”, “Pretty Much”, and “Very Much”). The DBD provides symptom scales for several disorders including two component scales under the ADHD symptom category: ADHD – Inattention (DBD-I) and ADHD – Hyperactivity/Impulsivity (DBD-H). Each of these scales has 9 items. This parent-report measure of inattention and hyperactivity has been used previously in studies of FASD and other developmental conditions (Antrop et al., 2002; Aragón et al., 2008; Kodituwakku et al., 2006). The dependent variables were the number of items endorsed (rated as “Pretty Much” or “Very Much”) for DBD-I and DBD-H. Because we were not using the DBD diagnostically, we used the continuous measure of the number of symptoms endorsed rather than the catetorical factor scores (criterion met or not met) in order to fully capture the variance in the parent ratings.
Neurobehavioral Evaluation System-3, A Continuous Performance Task (CPT)
The CPT (Letz, Green, & Woodard, 1996) is a computerized vigilance test, which requires each child to respond by pressing a button as quickly as possible in response to a target. This study utilized the “Following” condition: which requires a child to respond to a stimulus (a picture of an animal) only when followed by another picture of an animal (e.g., a cat when it follows a wolf). This measure provided an objective measure of inattention and has been used in other studies of typically developing children (Otto, Skalik, House, & Hudnell, 1996) and children with prenatal alcohol exposure (Mattson, Roesch, et al., 2010). The dependent variable was the number of omission errors on the Following condition.
MotionLogger Actigraphs (ACT)
Ambulatory Monitoring, Inc. MotionLogger Actigraphs are acceleration-sensitive devices that were worn on the non-dominant wrist of each child during an approximately 6-hour portion of a neuropsychological evaluation. Because the children wore the actigraph for slightly varying amounts of time due to the length of their individual test session, we selected the overall median activity across the first 5 hours of testing as the dependent variable to capture the largest sample. The wrist placement was used rather than the trunk location due to increased sensitivity to motion (Eaton, McKeen, & Saudino, 1996). The Proportional Integrating Measure (PIM) was used as a measure of the gross amount of motion detected by the actigraph. PIM is a digital actigraphic recording modality that integrates all signal information from the sensor to calculate the area under a rectified curve for each epoch, which maps onto the intensity of the movement or gross activity level (Alderson, Rapport, Kasper, Sarver, & Kofler, 2012; Tryon, 2005). The first analysis used the overall median of total movement activity, sampled 16 times per second throughout the entire session. The secondary analysis used individual medians for 30-minute time blocks to examine the data in a more detailed manner. This measure provided an objective measure of gross activity levels over time and has psychometric validity and reliability as demonstrated in other studies of ADHD (McGrath et al., 2004; Teicher et al., 1996).
2.4. Statistical Analyses
2.4.1. Demographic Information
All statistical analyses were conducted using the SPSS statistical package version 19.0 (SPSS, 2010). Demographic data were analyzed using standard analysis of variance (ANOVA) [age, BRIEF-IQ, and socioeconomic status as measured by the Hollingshead Index (SES)] and chi-square tests of independence [sex, race, ethnicity, and handedness]. Significant group differences on ANOVA were followed up using pairwise comparisons [Tukey’s Honestly Significant Difference (HSD) test]. Alpha levels for all analyses were set at .05.
2.4.2. Assessment of Covariates
We considered demographic variables that were associated with dependent variables at the p < .05 level as possible covariates. Sex was significantly correlated with DBD-H (r = −.328, p = .003). Age was significantly associated with the CPT (r = − .394, p < .001) and ACT (r = −.538, p < .001). Ethnicity, race, SES, and handedness were not correlated with CPT, ACT, DBD-H, or DBD-I, (ps > .151). Therefore, age and sex were included as covariates throughout the analysis. The Brief-IQ score, while only correlated with DBD-I (r = −.371, p = .001), was not considered a covariate due to inherent methodological and statistical issues including non-linear relationships with other cognitive domains and the inappropriateness of removing a variable associated with population representativeness (Dennis et al., 2009).
2.4.3. Laboratory Validation of Parent Reports and Group Differences
All variables (ACT, CPT, DBD-I, DBD-H) were standardized to z-scores based on the whole sample for statistically appropriate comparison between measures. A mixed-model repeated measures multivariate analysis of variance (MANCOVA) was conducted with Group as a between subject variable, and Domain (hyperactivity, inattention) and Type (laboratory, parent) as the within-subject variables. Appropriate covariates were included (sex, age). All significant interactions were followed up with simple main effects and pairwise analyses. For hyperactivity, the subjective measure was the parent-reported DBD Hyperactivity/Impulsivity scale score (DBD-H) and the objective laboratory measure was the Median Actigraphy Activity Level (ACT). For inattention, the subjective measure was the DBD Inattention scaled score (DBD-I) and the objective laboratory measure was the CPT Omission Errors scaled score (CPT). This analysis tested both the consistency of parent and lab measures across groups as well as group differences on all variables. Additional post-hoc analyses were conducted when warranted.
3. Results
3.1. Demographic Information
Groups did not differ significantly on age, sex, handedness, SES, race, and ethnicity (ps > .253). As expected, groups differed based on Brief-IQ score [F(2,82) = 5.617, p = .005]. Follow up analyses revealed that the AE group performed significantly worse than the CON group (p = .006), and no other group differences (AE = ADHD; ADHD = CON, ps >.256) were present. The Brief-IQ score did not correlate significantly with the outcome variables in the AE group (ps > .32). Demographic information presented in Table 1.
Table 1.
Demographic information for for the alcohol-exposed (AE), attentiondeficit/hyperactivity disorder (ADHD) and control (CON) groups.
| AE | ADHD | CON | |
|---|---|---|---|
| (n = 44) | (n = 16) | (n = 22) | |
| Variable | |||
| Handedness [n (% Right)] | 37 (84.1) | 15 (93.8) | 21 (95.5) |
| Sex [n (% Female)] | 15 (34.1) | 4 (25.0) | 11 (50.0) |
| Race [n (%White)] | 28 (63.6) | 11 (68.8) | 17 (77.3) |
| Ethnicity [n (% Hispanic)] | 5 (11.4) | 2 (12.5) | 5 (22.7) |
| Age [M (SD)] | 12.3 (3.52) | 12.2 (3.43) | 11.9 (2.71) |
| SES [M (SD)] | 46.8 (12.60) | 51.7 (9.96) | 49.5 (11.52) |
| IQ [M (SD)]* | 96.4 (16.25) | 101.4 (19.31) | 109.6 (12.08) |
| FAS Diagnosis [n (% FAS)] | 9 (20.5) | 0 (0.0) | 0 (0.0) |
| ADHD Diagnosis [n (% ADHD)] | 34 (77.3) | 16 (100.0) | 0 (0.0) |
| Predominately-Inattentive | 13 (38.2) | 10 (62.5) | 0 (0.0) |
| Hyperactive-Impulsive | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Combined | 21 (61.8) | 6 (37.5) | 0 (0.0) |
IQ estimate based on Leiter-R Brief-IQ score. Significant group differences were found on this measure with AE < ADHD and CON, p < .05.
3.2. Laboratory Validation of Parent Reports and Group Differences
Data for the four dependent variables are presented in Figure 1. There was a significant three-way interaction between Group × Type × Domain with age and sex as covariates [F(2,77) = 5.331, p = .007), η2 = .122]. To follow up this significant interaction, we conducted a 2-way analysis of Group × Type for each domain.
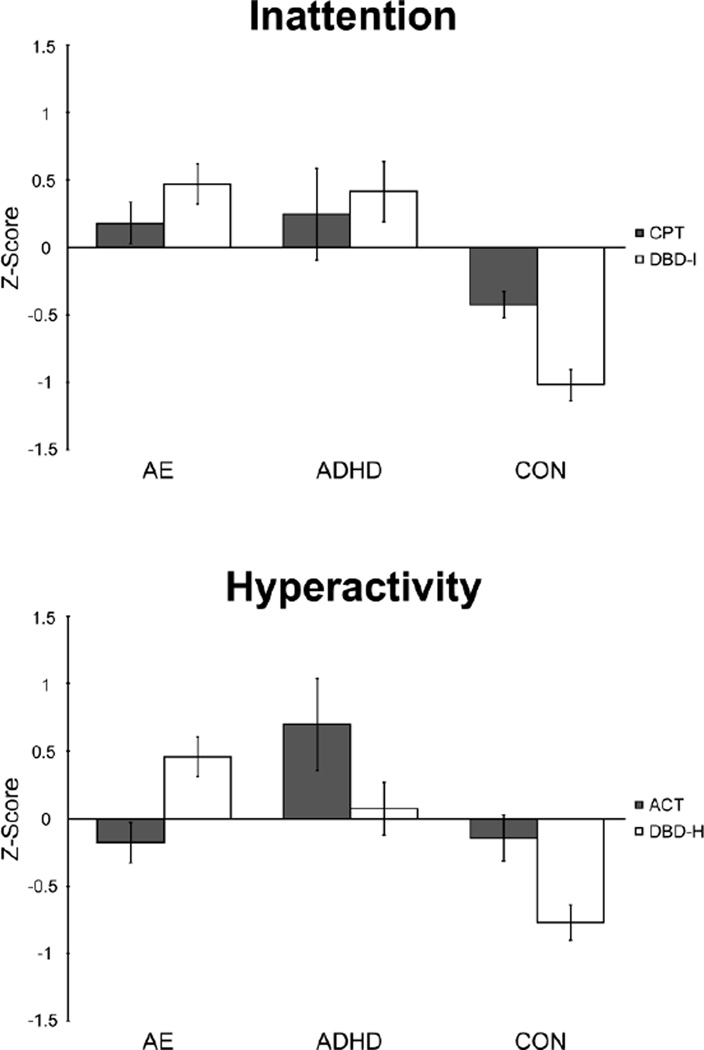
Figure 1.
Average scores for parent-reported and laboratory measures of inattention and hyperactivity for the alcohol-exposed (AE), attention-deficit/hyperactivity disorder (ADHD) and control (CON) groups. Positive scores indicate higher median activity for Actigraph (ACT), increased number of omission errors (CPT), and higher symptom counts for parent-reported hyperactivity and inattention (DBD-H, DBD-I). Individual subject data were converted to z-scores, based on the entire study sample and are presented as mean z-score +/− standard error.
3.2.1. Inattention
There was a significant Group × Type interaction, F(2,77) = 4.306, p = .017), partial η2 = .101. Simple effects were then used to examine Group at different levels of Type within the inattention domain. There was a significant main effect of Group for the parent measure of inattention (DBD-I), F(2, 77) = 29.353, p < .001, partial η2 = .433. Pairwise comparisons revealed that the clinical groups had significantly higher inattention scores compared to the control group (ps < .001), and did not differ from each other (p = .768). There was also a significant main effect of Group on the laboratory measure for inattention (CPT), F(2, 77) = 4.737, p = .011, partial η2 = .110. Follow up pairwise analyses revealed that the clinical groups had higher rates of inattention [AE (p = .003) and ADHD (p = .013)] compared to the control group, but did not differ from each other (p = .830).
3.2.2. Hyperactivity
There was a significant two-way interaction between Group × Type, F (2,77) = 12.658, p < .001), partial η2 = .247. Simple effects were then used to examine Group at each Type within the hyperactivity domain. There was a significant main effect of Group on the parent measure for hyperactivity (DBD-H), F(2, 77) = 15.114, p < .001, partial η2 = .282. Pairwise comparisons revealed that the clinical groups (AE, ADHD) had significantly higher scores compared to the control group (ps < .001), and did not differ from each other (p = .078). There was also a significant main effect of Group on the laboratory measure for hyperactivity (ACT), F(2, 77) = 7.313, p = .001, partial η2 = .160. Follow up analyses revealed that for the laboratory measure of hyperactivity (ACT), the ADHD group demonstrated higher activity compared to the AE (p = .001) and CON group (p = .002), which did not differ from each other (p = .937). To further ensure our findings are robust, we compared the actigraphy data of the AE and CON groups using the SPSS ANOVA bootstrapping technique and found no significant group differences after 1,000 iterations, F(1,65) = .021, p = .884, even after including age as a covariate, F(1,63) = .024, p = .877.
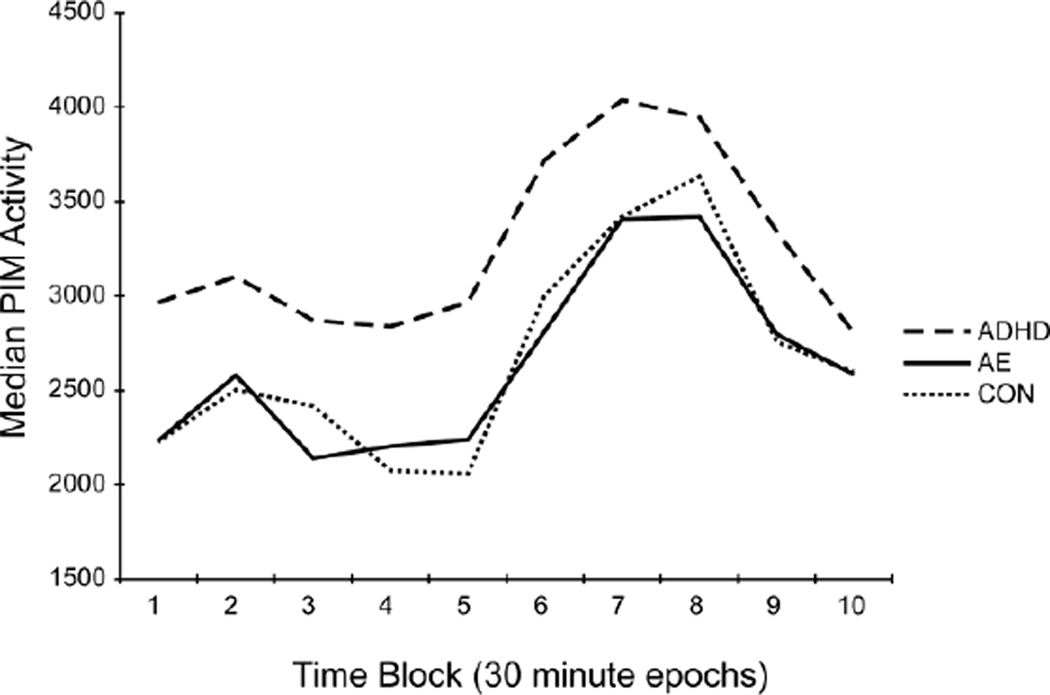
The discordance between parent and laboratory measures for hyperactivity prompted post-hoc analyses to further evaluate the differences on ACT across groups. This data was analyzed using repeated measures ANCOVA with 30-minute Time Blocks (Blocks 1 – 10) as the within-subject factor and Group (AE, ADHD, CON) as the between-subject factor. Age was included as a covariate and sex was included as a factor. Within-subjects tests were corrected using the Greenhouse-Geiser correction. There was no significant Group × Time Block interaction [F (12.574, 477.82) = 0.693, p = .766, partial η2 = .018). There was a significant main effect of Group [F(2, 76) = 5.237, p = .007, partial η2 = .121] and Time Block [F(6.287, 477.822) = 5.054, p < .001, partial η2 = .062]. Follow up pairwise analyses for Group revealed no significant differences in activity levels between AE and CON (p = .852). Consistent with the main analysis, activity in the ADHD group remained significantly higher than both the AE (p = .005) and CON (p = .001) groups. These results are illustrated in Figure 2.
Figure 2.
Median activity level as measured by actigraphy (laboratory measure of activity) over a 5-hour period across groups: children with prenatal alcohol exposure (AE), attention-deficit/hyperactivity disorder (ADHD), and controls (CON). Each time block is a 30-minute period.
3.2.3 Post-Hoc Power Analyses of Actigraph Data
To ensure that our lack of significant findings between the AE and CON groups on actigraphy is not due to sample size, we conducted additional post-hoc power analyses to determine the sample size needed to detect an effect. Using G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007), and the z-score means of the median activity level for AE (M: −0.1784, SD: 0.9943) and CON (M: −0.1427, SD: 0.8037), the calculated a priori effect size d was 0.0395. Using a two-tailed test with an alpha of .05, an allocation ratio of .5 (as CON has 22 subjects and AE has 44), and the methodologically appropriate and conventional power value 0.8, the total sample size required to detect an effect would be 22,652 (AE: 15,101, CON: 7,551). The need for such a large sample size to detect an effect indicates that our null finding is very likely due to a true null effect. This is compared to the ability to confirm equivalence between the means of the median activity on the actigraph for AE and ADHD (M: 0.6984, SD: 1.0403) groups, with an alpha of .05, an allocation ratio of .36 (16 ADHD/44 AE) and power of 0.8, the effect size d calculated by G*Power is 0.8616. The total sample size needed is 58 subjects (15 ADHD and 43 AE), which we exceeded and thus are confident in the increased activity level present in ADHD compared to AE.
3.2.4 Exploratory Analyses Omitting Alcohol-Exposed Subjects without ADHD
Given that our sample of alcohol-exposed children consisted of those with and without ADHD (44 AE total, 34 with ADHD), we repeated our analyses after omitting the 10 subjects from the AE group that did not meet criteria for ADHD. Results of these analyses were the same as those reported above, including the group differences in objectively measured activity (ADHD > AE, CON). Further, we repeated the bootstrapping analysis, described above, excluding AE subjects without ADHD and found no significant group differences, F(1,55) = .029, p = .865), even after including age as a covariate, F(1,53) = .063, p = .803).
4. Discussion
This study examined whether laboratory measures of hyperactivity and inattention were consistent with primary caregiver reports and if group differences existed on these measures. Parents of children in the clinical groups (AE, ADHD) reported elevated inattention and hyperactivity compared to controls (CON). For inattention, children in both clinical groups demonstrated significantly higher inattention on the objective laboratory measure (CPT), verifying the parent report. For hyperactivity, the laboratory measure of actigraphy (ACT) only supported the parent-reported hyperactivity in the ADHD population, but not in the AE group, which did not differ from controls.
4.1 Inattention
Children with histories of prenatal alcohol exposure and children with ADHD displayed significantly higher rate of omission errors, which distinguished them from typically developing controls. This confirms previous studies indicating that inattention is a shared clinical characteristic (Coles, Platzman, Lynch, & Freides, 2002; Kooistra et al., 2010; Lee, Mattson, & Riley, 2004; Streissguth et al., 1994). However, a recent systematic review (Dolan, Stone, & Briggs, 2010) of CPT tasks in children with prenatal alcohol exposure found inconclusive results with several studies reporting a significant association between alcohol exposure and omission errors (Coles et al., 2002; Lee et al., 2004; Streissguth et al., 1994), while others indicated no significant relation (e.g., Leech, Richardson, Goldschmidt, & Day, 1999). Discrepancies between studies on inattention may be due to differences in sample characteristics and degree (timing, dosage) of alcohol exposure.
Deficits in the underlying mechanisms of attention (e.g., information processing, general intelligence, and sluggish cognitive tempo), which have been reported in FASD (e.g., Coles et al., 1997; Glass et al., 2013; Graham et al., 2013), may also exacerbate inattention in this population, and future study should probe the individual components of inattention. Inattention may be more commonly reported in children with FASD who also meet criteria for ADHD compared to alcohol-exposed children without the additional diagnosis. Although our exploratory analyses omitting AE subjects without ADHD were consistent with our primary analyses, additional research is needed.
This finding of inattention in children with AE is clinically significant as attention deficits in children with FASD persist into adulthood and cause secondary disabilities (Fryer et al., 2007; Streissguth et al., 1994). Fortunately, effective, albeit limited, remediation is possible (Peadon, Rhys-Jones, Bower, & Elliott, 2009). The agreement between the laboratory measure (CPT) and parent-reported DBD indicates that DBD-I items may be useful in screening for general inattention in these populations.
4.2. Hyperactivity
In the domain of hyperactivity, there were parent-reported elevations in both clinical groups compared to controls. However, only the ADHD group demonstrated increased activity on the laboratory measure. Alcohol-exposed children, of whom 77.3% had a diagnosis of ADHD, did not differ from controls on actigraphy. The consistent elevations on both measures (DBD-H and actigraph) in the ADHD group support the sensitivity and validity of wrist-worn actigraphy to detect the cardinal feature of hyperactivity in that population.
Compared to controls, the ADHD group demonstrated a 24% increase in activity, as measured by the actigraph. This is comparable to previous studies on actigraphy found that compared to controls, children with ADHD are approximately 25 – 30% more active during academic classroom activities (Porrino et al., 1983) and laboratory-based attention tasks (Halperin et al., 1992). Thus, our findings further validate this measure further in a laboratory setting. These findings are supported by the extant literature indicating that behavioral issues found in FASD occur independently from the hyperactivity in children with ADHD (Peadon & Elliott, 2010) and that inattentive behaviors, but not hyperactivity, distinguish children with FASD from controls (Kodituwakku et al., 2006).
Differences in the outcomes between objective and parent-reported measures of hyperactivity in alcohol-exposed children may be due to the behaviors captured by the DBD-H. In addition to questions on hyperactivity, the DBD-H includes questions on impulsivity, although some items tap into both behaviors. Further, approximately a quarter of the AE group did not meet criteria for ADHD, which may have affected activity levels. However, our exploratory analyses omitting these subjects were consistent with our primary analyses and thus, our results are not likely due to the fact that ADHD was not present in the entire AE group. Variations in context may also partially explain the discrepancies within parent and laboratory measures as this study was conducted in a one-on-one structured setting. However, actigraphy has been found to discriminate ADHD from controls in both classroom and laboratory attention tasks suggesting that increased motor activity is independent from environmental change or cognitive demand (Halperin et al., 1992; Porrino et al., 1983). As this study did not include a classroom setting, it is not known whether the same would be true in the FASD population.
The results of this study indicate that children with AE may not display the same increased locomotor activity as children with ADHD. Our analysis of the patterns of activity using 30-minute blocks suggests similar patterns of activity across groups, albeit at different levels. As a result, these results provide clinically relevant information for school performance where children are less active during the earlier hours and most active midday. Such information could be utilized to facilitate effective and efficient structuring of school activities.
Overall, it appears that attention deficits are more robust than hyperactivity within the FASD population. As this is the first time that hyperactivity has been examined objectively in this population, we conducted additional analyses to confirm actigraphy findings in the alcohol-exposed population. Our post-hoc analysis across 30-minute intervals as well as our power and bootstrapping analyses support the lack of locomotor hyperactivity in the AE group.
4.3. Strengths and Limitations
This study has notable strengths including the novel design of comparing groups on subjective and objective measures of inattention and hyperactivity. It is the first study to use actigraphy to objectively measure levels of activity in children with FASD during the day. Despite having clinically relevant and significant findings, these results should be considered in the context of important limitations. The CIFASD Phase I sample is comprised of children recruited on a volunteer basis, although some subjects in both clinical groups were informed about our study in clinical settings. Further, subjects were from one CIFASD site only. These subject characteristics may influence generalizability. Further, all prenatal alcohol exposure information was determined retrospectively and the sample sizes, while yielding robust findings, are relatively small. However, we have used these recruitment strategies before with success and the current sample is consistent with the FASD population in as much as approximately 70% of the group has ADHD, and 20% has FAS. Post-hoc power analyses and bootstrapping indicates that there was adequate power to detect the effects and that these findings are robust.
We used actigraphs attached to the non-dominant wrist, not worn in pouches around the waist as in other studies of ADHD (Halperin et al., 1992). The placement of the actigraph may affect measurement of activity levels although it is inconclusive as to whether pouch or wrist placement reflects greater sensitivity to hyperactive behaviors. Placement of the actigraph in a pouch around the waist or head is more likely to detect gross body movements, however arm recordings detect more subtle movements and result in richer activity recordings (Eaton et al., 1996). Other methods of objective examination (such as infrared motion detection) could also provide useful data.
There may be a higher rate of alcohol-exposed children with ADHD - Inattentive subtype, compared to Hyperactive/Impulsive subtype as seen in other studies (Kodituwakku et al., 2006; O'Malley & Nanson, 2002). We did not have the power to examine differences between ADHD subtypes and further inquiry into the differences is warranted. Descriptively, most children in the ADHD group had Inattentive subtype (62.5%), while the rest had the Combined subtype (37.5%) whereas more children with ADHD in the AE group had the combined subtype (Inattentive, 38.2%, Combined, 61.8%).
There have also been reports of sex differences in the study of ADHD and locomotor hyperactivity. For example, animal studies (Hellemans et al., 2010) found that male animals pre-exposed to alcohol demonstrate locomotor hyperactivity, whereas alcohol-exposed females did not. Likewise in a study of children with FASD, boys were more likely to be diagnosed with ADHD (68%) compared to girls (29%), although there was no impact of sex or diagnosis on behavioral measures of attention (Herman et al., 2008). The current study findings accounted for the influence of sex and age, however future research in this area may be helpful in determining the prevalence of certain characteristics by sex and exposure history. Further, the distribution of ethnicity, while not significantly different across groups and not related to our outcome variables, may influence the results of behavioral problems in these groups and future study should continue to assess this as a potential covariate. Although alcohol-exposed children had lower IQ than control children, all groups were within the average range. The BRIEF IQ was not correlated with our behavioral outcome variables for the AE group and was not included as a potential covariate as it would reduce generalizability, is not related to cognitive domains in a linear fashion, and is not an appropriate covariate for studies of neurodevelopmental disorders (Dennis et al., 2009).
Medication use was not accounted for, which may have additional implications, especially because the clinical groups may be affected differently. While all children were asked to abstain from medication on test day, for some this was not feasible. On the day of assessment, 16 children in the AE group (36.4%) and 4 children in the ADHD (25.0%) group took ADHD medication. Parents reported that 20 children in the AE group (45.5%) and 7 children in the ADHD group (43.8%) regularly took ADHD medication. Medication may have differentially affected hyperactivity, but not inattention, in the AE group. However, this effect is unlikely as both inattention and hyperactivity were present in the ADHD group, despite almost equivalent rates of children in both clinical groups being medicated at time of testing and regularly prescribed medication.
4.4 Implications and Future Directions
Parent reported elevations of ADHD symptomology were validated by the objective measures of inattention in both children with heavy prenatal alcohol exposure and children with ADHD, but hyperactivity in the ADHD group alone. Only parent-reported inattention has been correlated with school achievement in the FASD population, and thus may have a more substantial influence on academic success (Aragón et al., 2008). Our findings suggest that actigraphy may be able to distinguish between alcohol-exposed children and children with idiopathic ADHD, as only the latter demonstrated significant elevations, despite the high rate of co-occurring ADHD in the AE group and previous anecdotal reports of hyperactivity (Landesman-Dwyer et al., 1981; Shaywitz et al., 1980). Replication of this study in a larger sample and comparisons of different ADHD subtypes are necessary to confirm these results.
These findings have implications for intervention and help explain some of the differential effects of pharmacological treatment. The differences in behavioral symptomatology for children with FASD, with inattention established as a potentially more salient feature than locomotor hyperactivity, may explain the discrepancy between children with ADHD and children with FASD in symptom resolution due to medication (Doig et al., 2008; Frankel, Paley, Marquardt, & O'Connor, 2006; Peadon et al., 2009). In one study, children with FASD and ADHD had higher parent-reported inattention scores compared to hyperactivity upon referral, and inattention was less responsive to medication indicating that it may be more prominent and require targeted treatment (Doig et al., 2008). Inattention also appears to account for more behavioral problems than hyperactive symptoms in alcohol-exposed children (Kodituwakku et al., 2006).
While these results suggest that parent reports of hyperactivity are not fully supported by more objective measures across groups, it may be that parent reports are capturing different behavioral facets of hyperactivity or impulsivity present in children with FASD. It is possible that the presentation of hyperactivity in children with FASD is unique when compared to children with ADHD, which may further facilitate identification and lend itself to different intervention techniques.
These results could also be used in a clinical setting to identify individuals who are likely to be alcohol-exposed. For example, the combination of high DBD scores and average activity scores would suggest that an individual child should be evaluated further for a history of prenatal alcohol exposure. Exploratory analysis of the current data suggests that such a model could be accurately used to identify alcohol-exposed individuals. Specifically, we classified subjects using cut-off scores for DBD inattention (raw scores >1) and actigraphy (PIM scores above 3508, which is 1 standard deviation above the sample average). When all three groups were considered together, 77.3% of the AE group and 86.4% of the CON group were accurately classified. However, the classification of the ADHD group (37.5%) did not differ from chance. To add further clinical utility to these analyses, three binary logistic regression analyses were conducted with the four dependent variables. When the AE and CON groups were included, overall classification accuracy was 89.4%, and 90.9% AE and 86.4% CON were correctly classified. DBD Inattention was a significant predictor. When the ADHD and CON groups were included, overall classification accuracy was 89.5%, and 81.3% ADHD and 95.5% CON were correctly classified. Actigraphy and DBD Inattention were significant predictors. Finally, when the AE and ADHD groups were included, overall classification accuracy was 76.7%, and 88.6% AE and 43.8% ADHD were correctly classified. Actigraphy and DBD Hyperactivity/Impulsivity were significant predictors. Interestingly, adding IQ to the third analysis did not improve accuracy. Thus it appears that children with histories of prenatal alcohol exposure can be accurately identified using these variables even when children with idiopathic ADHD are included. Children with ADHD however, may be inaccurately classified using this model and additional variables should be considered to improve specificity.
4.5. Conclusions
These results illustrate the challenges of parent and laboratory behavior documentation in children with FASD. We support the ongoing movement, evidenced by the DSM5, toward the use of a multi-method approach of diagnosis including objective performance measures of children to complement caregiver behavioral ratings forms. This methodology can better characterize behavioral symptomology and compensate for potential bias and subjectivity of a single source. Additional research should carefully inquire and assess the overlapping deficits among these related populations.
Supplementary Material
Table 2.
Repeated measures interactions and main effects of the between subjects factor of Group [alcohol-exposed (AE), attention-deficit/hyperactivity disorder (ADHD) and control (CON)]; and the within subjects factors of Domain (Inattention, Hyperactivity) and Type (Parent, Laboratory) on the four dependent variables (DBD-I, CPT, DBD-H, ACT) with sex and age as covariates.
| Effects | F | p | Partial η2 | Significant (p < .05) Pairwise Comparisons |
|---|---|---|---|---|
| Group×Type×Domain (2, 77) | 5.331 | .007 | .122 | --- |
| Group×Type for Inattention (2, 77) | 4.306 | .017 | .101 | ---- |
| Group for DBD-I (Inattention, Parent) (2, 77) | 29.353 | <.001 | .433 | AE, ADHD > CON ADHD = AE |
| Group for CPT (Inattention, Laboratory) (2, 77) | 4.737 | .011 | .110 | AE, ADHD > CON ADHD = AE |
| Group×Type for Hyperactivity (2, 77) | 12.658 | <.001 | .247 | --- |
| Group for DBD-H (Hyperactivity, Parent) (2, 77) | 15.114 | <.001 | .282 | AE, ADHD > CON ADHD = AE |
| Group for ACT (Hyperactivity, Laboratory) (2, 77) | 7.313 | .001 | .160 | AE = CON ADHD > AE, CON |
Highlights.
Concordance of objective and qualitative measures of inattention and hyperactivity.
Parent-report questionnaires compared to laboratory CPT and actigraphy.
ADHD and AE had increased inattention for laboratory and parent-report measures.
Parent-reported elevated hyperactivity in AE not objectively substantiated.
Differential behavioral profile between ADHD and AE for hyperactivity.
Acknowledgements
Research described in this paper was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant numbers U01 AA014834 (Mattson), U24 AA014811 (Riley), U24 AA014818 (Barnett), U24 AA014815 (Jones). Additional funding was provided through grants T32 AA013525 and F31 AA022261. The authors extend gratitude to the families and children who graciously participate in our studies and to the members of the Center for Behavioral Teratology for ongoing assistance and support, particularly the efforts of Jill Vander Velde, Andrew Vollmer, and Kristina Hubbard.
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the NIAAA. Additional information about CIFASD can be found at www.cifasd.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Alderson RM, Rapport MD, Kasper LJ, Sarver DE, Kofler MJ. Hyperactivity in boys with attention deficit/hyperactivity disorder (ADHD): The association between deficient behavioral inhibition, attentional processes, and objectively measured activity. Child Neuropsychology. 2012;18(5):487–505. doi: 10.1080/09297049.2011.631905. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-IV-TR. Washington, DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-5. 5th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- Antrop I, Roeyers H, Oosterlaan J, Van Oost P. Agreement between parent and teacher ratings of disruptive behavior disorders in children with clinically diagnosed ADHD. Journal of Psychopathology and Behavioral Assessment. 2002;24(1):67–73. [Google Scholar]
- Aragón AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Gossage JP, May PA. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2008;32(11):1909–1919. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R, Murphy K. Attention-Deficit Hyperactivity Disorder: A Clinical Workbook. 2 ed. New York: Guilford Press; 1998. [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC, Grossbard JR. How informative are parent reports of attention-deficit/hyperactivity disorder symptoms for assessing outcome in clinical trials of long-acting treatments? A pooled analysis of parents' and teachers' reports. Pediatrics. 2004;113(6):1667–1671. doi: 10.1542/peds.113.6.1667. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2002;26(2):263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21(1):150–161. [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig J, McLennan JD, Gibbard WB. Medication effects on symptoms of attention-deficit/hyperactivity disorder in children with fetal alcohol spectrum disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18(4):365–371. doi: 10.1089/cap.2007.0121. [DOI] [PubMed] [Google Scholar]
- Dolan GP, Stone DH, Briggs AH. A systematic review of continuous performance task research in children prenatally exposed to alcohol. Alcohol and Alcoholism. 2010;45(1):30–38. doi: 10.1093/alcalc/agp062. [DOI] [PubMed] [Google Scholar]
- Dupaul GJ. Parent and Teacher Ratings of Adhd Symptoms - Psychometric Properties in a Community-Based Sample. Journal of Clinical Child Psychology. 1991;20(3):245–253. [Google Scholar]
- Eaton WO, McKeen NA, Saudino KJ. Measuring human individual differences in general motor activity with actometers. In: Ossenkopp K-P, Kavaliers M, Sanberg PR, editors. Measuring movement and locomotion: From invertebrates to humans. Austin, TX: R.G. Landes Company; 1996. pp. 79–92. [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Frankel F, Paley B, Marquardt R, O'Connor M. Stimulants, neuroleptics, and children's friendship training for children with fetal alcohol spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2006;16(6):777–789. doi: 10.1089/cap.2006.16.777. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119(3):e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Glass L, Ware AL, Crocker N, Deweese BN, Coles CD, Kable JA the CIFASD. Neuropsychological deficits associated with heavy prenatal alcohol exposure are not exacerbated by ADHD. Neuropsychology. 2013 Sep 16; doi: 10.1037/a0033994. (2013) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Crocker N, Deweese BN, Roesch SC, Coles CD, Kable JA the CIFASD. Prenatal alcohol exposure, attention-deficit/hyperactivity disorder, and sluggish cognitive tempo. Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E338–E346. doi: 10.1111/j.1530-0277.2012.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Matier K, Bedi G, Sharma V, Newcorn JH. Specificity of inattention, impulsivity, and hyperactivity to the diagnosis of attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):190–196. doi: 10.1097/00004583-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Rhee SH, Willcutt EG, Pennington BF. Modeling rater disagreement for ADHD: Are parents or teachers biased? Journal of Abnormal Child Psychology. 2007;35(4):536–542. doi: 10.1007/s10802-007-9110-y. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive-and anxiety-like behaviors in male and female offspring. Alcoholism: Clinical and Experimental Research. 2010;34(4):633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang P-N. Gender and attention deficits in children diagnosed with a fetal alcohol spectrum disorder. The Canadian Journal of Clinical Pharmacology. 2008;15(3):e411–e419. [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6):e1734–e1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragón AS, Kalberg WO, Buckley D, May PA. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: Preliminary results. Alcoholism: Clinical and Experimental Research. 2006;30(9):1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Kaplan BJ, Fan J. Comparing attentional networks in fetal alcohol spectrum disorder and the inattentive and combined subtypes of attention deficit hyperactivity disorder. Developmental Neuropsychology. 2011;36(5):566–577. doi: 10.1080/87565641.2010.549978. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2010;52(2):205–211. doi: 10.1111/j.1469-8749.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- LaDue RA, Streissguth AP, Randels SP. Clinical considerations pertaining to adolescents and adults with fetal alcohol syndrome. In: Sonderegger TB, editor. Perinatal Substance Abuse: Research Findings and Clinical Implications. Baltimore, MD: The Johns Hopkins University Press; 1992. pp. 104–131. [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, Shaffer D. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. The American Journal of Psychiatry. 1994;151(11):1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Landesman-Dwyer S, Ragozin AS, Little RE. Behavioral correlates of prenatal alcohol exposure: A four-year follow-up study. Neurobehavioral Toxicology and Teratology. 1981;3(2):187–193. [PubMed] [Google Scholar]
- Landgren M, Svensson L, Strömland K, Andersson Grönlund M. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics. 2010;125(5):e1178–e1185. doi: 10.1542/peds.2009-0712. [DOI] [PubMed] [Google Scholar]
- Lee KT, Mattson SN, Riley EP. Classifying children with heavy prenatal alcohol exposure using measures of attention. Journal of the International Neuropsychological Society. 2004;10(2):271–277. doi: 10.1017/S1355617704102142. [DOI] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicology and Teratology. 1999;21(2):109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Letz R, Green RC, Woodard JL. Development of a computer-based battery designed to screen adults for neuropsychological impairment. Neurotoxicology and Teratology. 1996;18(4):365–370. doi: 10.1016/0892-0362(96)00041-4. [DOI] [PubMed] [Google Scholar]
- Letz R, Pieper WA, Morris RD. NES test performance in a large US Army veteran sample: Relationships with both demographic factors and traditional neuropsychological measures. Neurotoxicology and Teratology. 1996;18(4):381–390. doi: 10.1016/0892-0362(96)00023-2. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20(3):361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund Å the CIFASD. Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol. 2010;44(7–8):635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. The quest for a neurobehavioral profile of heavy prenatal alcohol exposure. Alcohol Research and Health. 2011;34(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund Å, Autti-Rämö I, Jones KL, May PA the CIFASD. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2010;34(9):1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental Disabilities Research Reviews. 2009;15(3):176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McGrath AM, Handwerk ML, Armstrong KJ, Lucas CP, Friman PC. The validity of the ADHD section of the Diagnostic Interview Schedule for Children. Behavior Modification. 2004;28(3):349–374. doi: 10.1177/0145445503258987. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- O'Malley KD, Nanson J. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2002;47(4):349–354. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- Otto DA, Skalik I, House DE, Hudnell HK. Neurobehavioral Evaluation System (NES): Comparative performance of 2nd-, 4th-, and 8th-grade Czech children. Neurotoxicology and Teratology. 1996;18(4):421–428. doi: 10.1016/0892-0362(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Peadon E, Elliott EJ. Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: Clinical guidelines. Neuropsychiatric Disease and Treatment. 2010;6:509–515. doi: 10.2147/ndt.s7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon E, Rhys-Jones B, Bower C, Elliott EJ. Systematic review of interventions for children with fetal alcohol spectrum disorders. BMC Pediatrics. 2009;9(35) doi: 10.1186/1471-2431-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Greenhill LL, Crimson ML, Sedillo A, Carlson C, Conners CK Texas Conference Panel on Medication Treatment of Childhood, A. The Texas Children's Medication Algorithm Project: Report of the Texas Consensus Conference Panel on Medication Treatment of Childhood Attention-Deficit/Hyperactivity Disorder. Part I. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(7):908–919. doi: 10.1097/00004583-200007000-00021. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Rapoport JL, Behar D, Sceery W, Ismond DR, Bunney WE., Jr A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Archives of General Psychiatry. 1983;40(6):681–687. doi: 10.1001/archpsyc.1983.04390010091012. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter international performance scale-revised: Examiner's manual. Wood Dale, IL: Stoelting Co.; 1997. [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Cohen DJ, Shaywitz BA. Behavior and learning difficulties in children of normal intelligence born to alcoholic mothers. Journal of Pediatrics. 1980;96(6):978–982. doi: 10.1016/s0022-3476(80)80621-4. [DOI] [PubMed] [Google Scholar]
- Sims DM, Lonigan CJ. Multi-method assessment of ADHD characteristics in preschool children: Relations between measures. Early Childhood Research Quarterly. 2012;27(2):329–337. doi: 10.1016/j.ecresq.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr H-L, Willms J, Steinhausen H-C. Fetal alcohol spectrum disorders in young adulthood. Journal of Pediatrics. 2007;150(2):175–179. doi: 10.1016/j.jpeds.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Sprafkin J, Volpe RJ, Gadow KD, Nolan EE, Kelly K. A DSM-IV-referenced screening instrument for preschool children: The Early Childhood Inventory-4. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(5):604–612. doi: 10.1097/00004583-200205000-00018. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS 19.0 for Mac OS X. Chicago, IL.: 2010. [Google Scholar]
- Streissguth AP. Fetal alcohol syndrome: Early and long-term consequences. In: Harris L, editor. Problems of drug dependence 1991: Proceedings of the 53rd annual scientific meeting (NIDA Research Monograph No.119) Rockville, MD: U.S. Department of Health and Human Services; 1992. pp. 126–130. [PubMed] [Google Scholar]
- Streissguth AP, Sampson PD, Olson HC, Bookstein FL, Barr HM, Scott M, Mirsky AF. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring -- a longitudinal prospective study. Alcoholism: Clinical and Experimental Research. 1994;18(1):202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Barber NI. Objective measurement of hyperactivity and attentional problems in ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(3):334–342. doi: 10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Lowen SB, Polcari A, Foley M, McGreenery CE. Novel strategy for the analysis of CPT data provides new insight into the effects of methylphenidate on attentional states in children with ADHD. Journal of Child and Adolescent Psychopharmacology. 2004;14(2):219–232. doi: 10.1089/1044546041648995. [DOI] [PubMed] [Google Scholar]
- Tryon WW. The reliability and validity of two ambulatory monitoring actigraphs. Behavior Research Methods. 2005;37(3):492–497. doi: 10.3758/bf03192719. [DOI] [PubMed] [Google Scholar]
- Ware AL, O'Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD the CIFASD. The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. Alcoholism: Clinical and Experimental Research. 2013;37(3):507–516. doi: 10.1111/j.1530-0277.2012.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengel T, Hanlon-Dearman AC, Fjeldsted B. Sleep and sensory characteristics in young children with fetal alcohol spectrum disorder. Journal of Developmental and Behaviorial Pediatrics. 2011;32(5):384–392. doi: 10.1097/DBP.0b013e3182199694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.