Abstract
Background:
Arsenic is a wide spread environmental contaminant and has been recognized as a genotoxic element which is of major public health concern.
Aim:
The present study evaluates the genotoxic potential of arsenic at low permissible dose levels.
Materials and Methods:
Forty-eight mature female rats were divided into four groups of 12 animals each. Group I animals received distilled water and served as control. Group II-IV animals received sodium arsenite dissolved in distilled water continuously for a period of 60 days at the dose of 10, 30 and 50 μg/L (ppb) respectively. Six rats from each group were sacrificed after 30 days of arsenic exposure and the remaining animals were sacrificed after 60 days. Liver was excised from the sacrificed animals to study the probable advent signs of carcinogenicity measured through microsomal degranulation test. Assessment of mutagenic potential of arsenic was evaluated through chromosomal aberrations observed in the bone marrow cells.
Results:
The levels of RNA and proteins decreased significantly (P ≤ 0.01) in all the three doses administered along with an increase in % microsomal degranulation in hepatic fraction when compared to control at both 30 and 60 days time period. A dose-dependent increase in chromosome aberrations like fragmentation, breakage has been observed in all the treated animals.
Conclusion:
The results of present study revealed that chronic exposure of arsenic even at its low permissible dose limits results in carcinogenic and mutagenic effects which emphasize its genotoxic possibility.
Keywords: Chromosome aberrations, genotoxic, microsomal degranulation, sodium arsenite
INTRODUCTION
The presence of arsenic in the environmental media results from both geogenic sources and anthropogenic activities. The occurrence of high concentrations of arsenic in ground water used for drinking purpose has been recognized as a major public-health concern in several parts of the world. Every day millions of people are exposed to arsenic via drinking water where the concentration of arsenic exceeds the permissible limit (10 μg/L) defined by the World Health Organisation (WHO).[1] Arsenic occurs in ground water in the form of arsenite, arsenate, methyl arsenic acid and dimethyl arsenic acid. Groundwater is predominately used for irrigation of agricultural crops which results in deposition of arsenic in crops and is the second largest contributor to arsenic uptake by people. Other potential sources of arsenic toxicity include the use of arsenic-contaminating herbicides, insecticides, rodenticides, preservatives and by products of fossil fuels.[2] Inhalation or ingestion of inorganic arsenic has been shown to cause cancer in humans, resulting in tumors of the skin, lung, liver, urinary bladder, and other locations, and has been classified as a proven human carcinogen by the International Agency for Research on Cancer (IARC),[3] in the EU (European Chemicals Bureau),[4] as well as by the US Environmental Protection Agency (EPA).[5] It has been reported that sub-chronic exposure to arsenic through drinking water alters the expression of cancer-related genes in liver,[6] increased the incidences of chromosome aberrations, sister chromatid exchanges and micronuclei in human populations.[7,8,9] Arsenic is not a direct DNA mutagen, but it diminishes DNA repair capacity and alters the DNA methylation patterns.[10] Existence of arsenic in different inorganic and organic forms, complicates its considerations on toxic effects. Toxicity varies according to its oxidation state, solubility and many other factors including the exposure dose, frequency, duration, species, age, gender, as well as individual susceptibilities, genetic and nutritional factors.[11,12,13] The well-known toxic effects of arsenic on human are difficult to reproduce in experimental animals,[14] but despite of this, the toxicological significance of low level oral exposure to arsenic and the dose response relationship for carcinogenic effects has been the subject of important discussion.
Although several in vitro studies have reported the genotoxic effects (carcinogenesis and mutagenesis) of arsenic at higher doses, the purpose of the present study is to focus on the evidence whether arsenic is capable of inducing/initiating genotoxic effects at low dose levels (10-50 μg/L) measured through hepatic microsomal degranulation and chromosomal aberration in bone marrow cells using female albino rats as an experimental model.
MATERIALS AND METHODS
Chemicals
Sodium arsenite and other chemicals used in the present study were purchased from S.D. Fine Chem. Ltd and were of analytical grade (AR).
Animals and experimental design
Forty-eight mature female rats were procured from Department of Livestock Production and Management, Guru Angad Dev Veterinary and Animal Sciences University (GADVASU), Ludhiana, and acclimatized for 15 days before using them for experimentation. The rats were maintained under controlled condition of temperature (27 ± 2°C; 12h light/dark cycles) and provided with standard pellet diet and water ad libitum. The rats were divided randomly into 4 groups consisting of 12 animals each. Group I animals received distilled water and served as control. Group II, III and IV animals received arsenic as sodium meta arsenite at doses of 10, 30 and 50 μg/L(ppb) dissolved in distilled water for a period of 60 days. Half of the animals (6) from each group were sacrificed after 30 days of arsenic exposure and remaining others after 60 days.
Chromosome aberration assay
Experimental animals were injected (intraperitonealy) with colchicine (4 mg/kg) 1.5 h prior to sacrifice and cytogenetic analysis was performed on bone marrow cells.[15] Both femora were dissected out and cleaned of any adhering muscle. Bone-marrow cells were collected from both femora by flushing in KCL (0.075 M, at 37°C) and incubated at 37°C for 25 min. Collected cells were centrifuged at 3000 rpm for 10 min, and fixed in aceto-methanol (acetic acid:methanol, 1:3, v/v). Centrifugation and fixation were repeated five times at an interval of 20 min. The cells were resuspended in a small volume of fixative, dropped onto chilled slides, dried and stained the following day with freshly prepared 2% Giemsa stain for 3-5 minutes.
Microsomal degranulation assay
Liver (0.5 gram) was finely chopped and homogenized in 0.225 M sucrose tris (ST) buffer (pH 7.4) in chilled conditions and processed for microsomal degranulation.[16,17] Tissue homogenates were centrifuged for 20 min at 9000 rpm at 4°C, the post mitochondrial supernatant collected and mixed with 0.5 g calcium chloride. After that the tubes were kept in ice for 20 min, centrifuged at 4°C, 10,000 rpm for 20 min. The pelleted microsomes were resuspended in 0.225 M ST buffer (pH 7.4) and proteins, RNA were estimated as per the standard methods. Microsomal degranulation values above 5% were taken as positive result for representing carcinogenic properties of the chemical.[18]
Statistical analysis
Statistical analysis of the data for microsomal degranulation test was carried out by one-way analysis of variance (ANOVA). The values of treated rats were compared with control and the statistical differences were considered significant at P ≤ 0.05, P ≤ 0.01. All values were expressed as mean ± SEM.
RESULTS
Microsomal degranulation test
The observations recorded indicate that exposure of arsenic at low permissible dose limits is capable of inducing microsomal degranulation [Table 1]. The exposure to arsenic both for 30 and 60 days results in a significant decrease (P ≤ 0.01) in RNA and proteins of treated rats when compared to control. Similarly, a dose-dependent increase in % degranulation has been observed in treated rats at both time periods of arsenic exposure. After 30 days of arsenic exposure, only 50 ppb (10.91%) and 30 ppb (7.43%) doses induced carcinogenic effects while 10 ppb dose caused only 4.51% degranulation which is considered as non-carcinogenic. However, all the three doses administered (50, 30 and 10 ppb) showed carcinogenic potential with per cent microsomal degranulation values of 14.04%, 7.60% and 5.82% respectively after 60 days of exposure [Table 1]. Hepatic fractions from the control group of rats showed 1.08% and 2.04% degranulation respectively for 30 and 60 days and were considered as non-carcinogenic.
Table 1.
Effect of sodium arsenite on hepatic microsomal degranulation in female rats

Chromosomal aberrations
A significant increase (P ≤ 0.01) in the chromosomal aberration in treated rats as compared to control animals indicating mutagenic behavior of arsenic [Table 2]. Structural chromosomal aberrations observed after exposure of arsenic were in the form of chromatid breakage (fragments, breaks and gaps). A dose-dependent increase in chromosomal aberrations was observed [Figure 1].
Table 2.
Assessment of chromosomal aberrations in the bone marrow cells of female rats after exposure to sodium arsenite
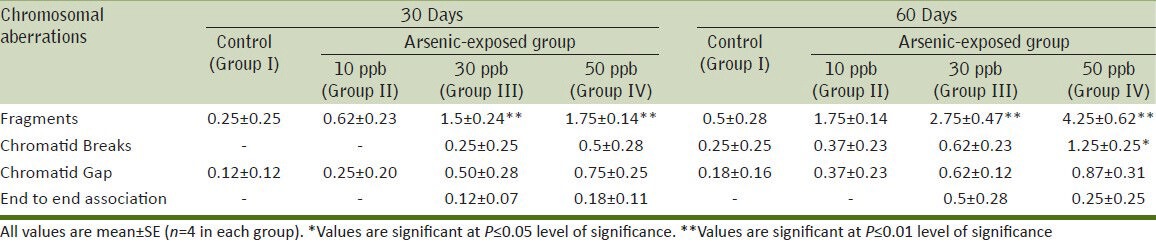
Figure 1.

Photograph showing different types of chromosomal aberration. (a) Control rats, 30 days; (b) Control rats, 60 days showing normal metaphase plate. (c) 10 ppb, 30 days; (d) 10 ppb, 60 days showing fragmentation, chromatid gap and breakage. (e) 30 ppb, 30 days; (f) 30 ppb, 60 days show fragmentation, chromatid break and end to end association of chromosomes. (g) 50 ppb, 30 days; (h) 50 ppb, 60 days show fragmentation and chromatid breaks. Red arrow showing fragmentation, Red dotted arrow showing chromatid gap, Black arrows showing breakage and Yellow arrow showing end to end association
DISCUSSION
In the present study the carcinogenic potential of arsenic was assessed by measuring the detachment of ribosomes from rough endoplasmic reticulum (RER). Earlier studies have reported that carcinogens degranulated RER under in vivo and in vitro conditions[19] resulting in a decreased RNA/Protein ratio and provides the basis of a screening test for environmental or chemical carcinogens.[20] Liver provides a good model for the study of carcinogen-induced degranulation, mainly for two reasons: firstly, it was a rich source of rough endoplasmic reticulum and secondly, it has the metabolic capacity required to generate active forms of carcinogen from precursors. The administration of arsenic consecutively for 30 and 60 days in the present study resulted in a decreased RNA/Protein ratio[20] which has been taken as an index of degranulation. Our results are in consonance to the earlier findings[21] where a decrease in RNA/Protein ratio of treated rats due to direct membrane degranulation has been reported. Researchers have demonstrated that electrophiles of a carcinogen can disrupt ribosome membrane interaction in rough microsomes by their attack on nucleophilic components of the reticular membrane ribosome complex, involved in protein synthesis for export from cytosol.[22] Lack of exported proteins can adversely affect signal transduction across plasma membrane possibly leading to events at molecular levels leading to incidence of carcinogenesis.
Arsenic has been recorded as a genotoxic element and not a mutagen for both animals and humans.[3,7] The significant chromosomal aberrations observed in the present study were mainly in the form of chromatid breakages (gaps, break and fragments) which support the view of genotoxicity expressed earlier. Various in vitro studies revealed that arsenic can damage DNA and induces the formation of chromosome aberrations, micronuclei formation and sister chromatid exchange in mammalian cells.[14,23] Chromatid lesions occur only when chromosomes are damaged after G1 stage of the cell cycle leading to chromatid breakage.[24] Cytogenetic studies done earlier showed that arsenic exposure has a positive genotoxic effect and an increased number of chromosomal aberrations on human lymphocytes.[25,26]
CONCLUSION
Chronic exposure of arsenic even at its low and permissible dose limits (10-50 μg/L) results in degranulation and chromosome aberrations which substantiates the possible genotoxic potential of arsenic in animals. However, further studies on animals are needed to hypothesize the detailed molecular mechanism involved in genotoxicity of arsenic laden compounds.
ACKNOWLEDGMENTS
The authors are thankful to the Head, Department of Zoology and Dean, College of Basic Sciences and Humanities, Punjab Agricultural University, Ludhiana, India for providing necessary facilities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.2nd ed. Vol. 1. Geneva: World Health Organization; 1993. WHO. Guidelines for drinking water quality: Recommendations. [Google Scholar]
- 2.Flora SJ, Mehta A, Rao PV, Kannan GM, Bhaokar A, Dube SN, et al. Therapeutic potential of monoisoamyl and monomethyl esters of meso 2,3-dimercaptosuccimic acid in gallium arsenide intoxicated rats. Toxicology. 2004;195:127–46. doi: 10.1016/j.tox.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3.IARC. International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans. [Last accessed on 31 Jan 2012];Some drinking-water disinfectants and contaminants, including arsenic. 2004 84:39. Available from: http://www.inchem.org/documents/iarc/vol84/84-01-arsenic.html . [PMC free article] [PubMed] [Google Scholar]
- 4.European Chemicals Bureau (ECB). European Chemical Substances Information System [ESIS] [database on the Internet]. c1995-2010. [cited 2010 October] Available from: http://ecb.jrc.ec.europa.eu/esis .
- 5.Environmental Protection Agency. Integrated Risk Information system (IRIS) [Last accessed on 2010 Oct 25];Toxicological Review of Inorganic Arsenic (Cancer) (2010 External Review Draft) [Google Scholar]
- 6.Cui XS, Shraim A, Kobayashi Y. Subchronic exposure to arsenic through drinking water alters expressions of cancer related genes in rat liver. Toxicol Pathol. 2004;32:64–72. doi: 10.1080/01926230490261348. [DOI] [PubMed] [Google Scholar]
- 7.Basu A, Mahata J, Roy AK, Sarkar JN, Poddar G, Nandy AK, et al. Enhanced frequency of micronuclei in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat Res. 2002;516:29–40. doi: 10.1016/s1383-5718(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 8.Lerda D. Sister chromatid exchange (SCE) among individuals chronically exposed to arsenic in drinking water. Mutat Res. 1994;312:111–20. doi: 10.1016/0165-1161(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 9.Gradecka G, Palus J, Wasowicz W. Selected mechanisms of genotoxic effects of inorganic arsenic compounds. Int J Occup Med Environ Health. 2001;14:317–28. [PubMed] [Google Scholar]
- 10.Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–61. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal B, Lucky TD. New York: Plenum Press; 1978. Metal toxicity in mammals. [Google Scholar]
- 12.Marafante E, Vahter M. Solubility, retention, and metabolism of intratracheally and orally administered inorganic arsenic compounds in the Hamster. Environ Res. 1987;42:72–82. doi: 10.1016/s0013-9351(87)80008-7. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Lin LJ. Human carcinogenicity and atherogenicity induced by chronic exposure to inorganic arsenic. In: Nriagu JO, editor. Arsenic in the Environment; Part II: Human Health and Ecosystem Effects. New York (NY): John Wiley and Sons, Inc; 1994. pp. 109–31. [Google Scholar]
- 14.Basu A, Mahata J, Gupta S, Giri AK. Genetic toxicology of a paradoxical human carcinogen, arsenic: A review. Mutat Res. 2001;488:171–94. doi: 10.1016/s1383-5742(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 15.Sharma AK, Sharma A. Singapore: Harwood Academic Publishers; 1994. Chromosome techniques - A manual. [Google Scholar]
- 16.Kamath SA, Naragan KA. Interaction of Ca2+ with endoplasmic reticulum of rat liver standardized procedure for the isolation of rat liver microsomes. Anal Biochem. 1972;48:53–61. doi: 10.1016/0003-2697(72)90169-8. [DOI] [PubMed] [Google Scholar]
- 17.Gupta MM, Dani HM. Standardized procedure for the isolation of rat liver microsomes. Indian J Exp Biol. 1979;17:11–44. [Google Scholar]
- 18.Purchase IFH, Jongstoff E, Ahby J, Styles JA, Lefevre PA, Westwood FR. An evaluation of 6 short term tests for detecting organic chemical carcinogens. Br J Cancer. 1978;37:873–903. doi: 10.1038/bjc.1978.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams DJ, Rabin BR. Disruption by carcinogens of the hormone dependent association of membranes with polysomes. Nature. 1971;232:102–5. doi: 10.1038/232102a0. [DOI] [PubMed] [Google Scholar]
- 20.Jagota SK, Dani HM. Microsomal degranulation by isatin and its inhibitiors by ascorbic acid. Curr Sci. 1981;50:721. [Google Scholar]
- 21.Orrenius S, Ericsson JL. Enzyme- membrane relationships in phenobarbital induction of synthesis of drug metabolising enzyme system and proliferation of endoplasmic reticulum. J Cell Biol. 1966;28:181. doi: 10.1083/jcb.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dani HM, Kaur P. Optical visualization of microsomal degranulation by a carcinogen. J Environ Pathol Toxicol Oncol. 2001;20:109–11. [PubMed] [Google Scholar]
- 23.Gebel TW, Liester M, Schumann W, Hirsch EK. Low level self tolerance to arsenite in human HepG2 cells is associated with a depressed induction of micronuclei. Mutat Res. 2002;514:245–55. doi: 10.1016/s1383-5718(01)00343-6. [DOI] [PubMed] [Google Scholar]
- 24.Febrer E, Mestres M, Caballín MR, Barrios L, Ribas M, Gutiérrez- Enríquez S, et al. Mitotic delay in lymphocytes from BRCA1 heterozygotes unable to reduce the radiation-induced chromosomal damage. DNA Repair (Amst) 2008;7:1907–11. doi: 10.1016/j.dnarep.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Basu A, Ghosh P, Das JK, Banerjee A, Ray K, Giri AK. Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in West Bengal, India: A comparative study in 3 cell types. Cancer Epidemiol Biomarkers Prev. 2004;13:820–7. [PubMed] [Google Scholar]
- 26.Ghosh P, Banerjee M, Chaudhari S, Das JK, Sarma N, Basu A. Increased chromosome aberration frequencies in Bowen's patients compared to non-cancerous skin lesions in individuals exposed to arsenic. Mutat Res. 2007;632:104–10. doi: 10.1016/j.mrgentox.2007.05.005. [DOI] [PubMed] [Google Scholar]


