Figure 4. There is no significant difference in tau kinase and phosphatase activities between wild-type and Nrf2 (−/−) mice.
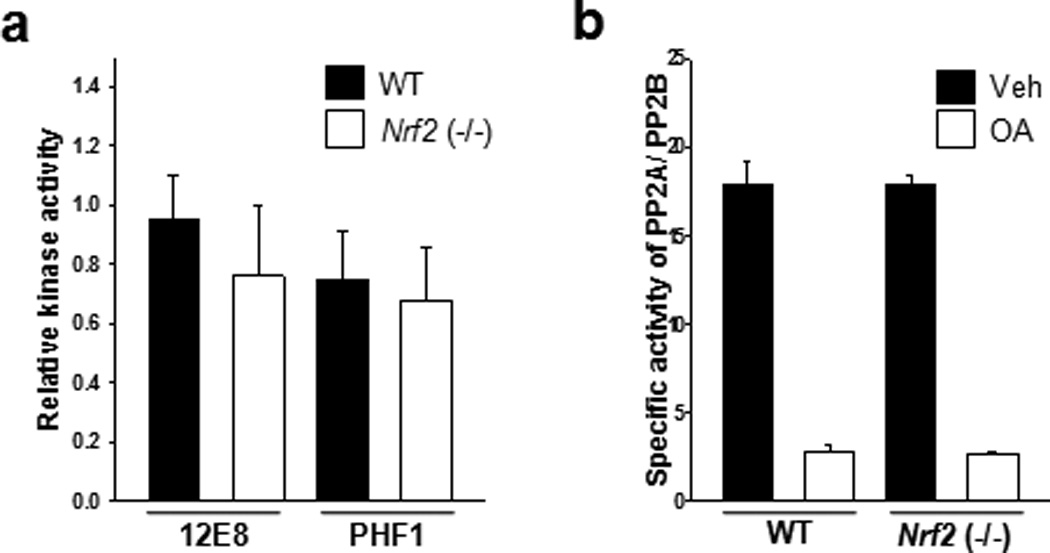
Mouse brains were obtained from wild-type (11 months old, 1 male; 5 months old, 1 female; 12 months old, 2 female) and Nrf2 (−/−) (10 months old, 2 male; 2 female) mice. (a)Mouse brain tissues were homogenized in the lysis buffer, and 10 µg of lysates was incubated with 2 µg of GST-tau protein at 37°C for 30 min in the presence or absence of 1 mM ATP. The levels of tau phosphorylated at Ser262/Ser356 and Ser396/Ser404 were analyzed by immunoblotting using a 12E8- and PHF1-specific antibody, respectively. GST-tau was detected with a monoclonal GST antibody (GST-tau) (Supplementary Fig. 4). Bar graph of the relative optical density of phosphorylated tau normalized to actin. Data shown are mean±SE of duplicated independent experiments and were analyzed using Student’s t test. (b) Mouse brain tissues were homogenized in the phosphatase storage buffer, and phosphatase activity in the lysates was quantitated using the Serine/Threonine Phosphatase Assay System (Promega) by measuring the dephosphorylation of a phospho-peptide, RRA(pT)VA in the presence or absence of okadaic acid (OA, 20 nM). PP2A activity was defined as the activity inhibited by the addition of okadaic acid to the phosphatase reaction mixture. n=4. Data shown are mean±SE of three independent experiments and were analyzed using Student’s t test.
