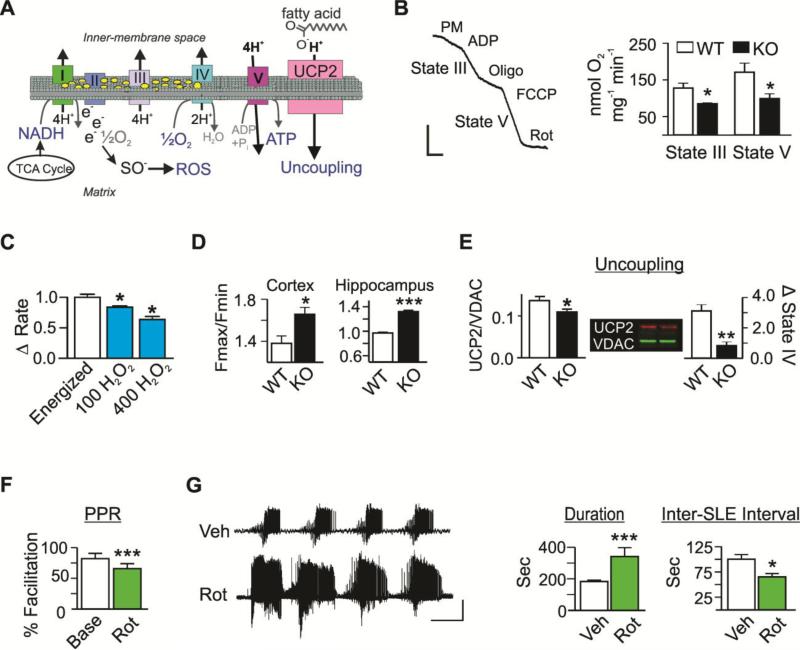
Figure 1. Impairment of mitochondrial functions is associated with epilepsy in vivo and hyperexcitable hippocampal circuitry in vitro.
(A) Schematic depicting oxidative phosphorylation, the binding of free electrons to molecular oxygen to form ROS, and fatty acid-mediated proton transport thru uncoupling protein (UCP) 2. (B) Raw WT oxygen polarographic trace illustrating respiratory rates and quantification of state III and V respiratory rates by WT (white) and KO (black) cortical mitochondria. Bars: 25 nmol O2 min −1 × 50 sec (n=5-7) (C) The change in stabilized state III respiration following sequential application of 100 and 400 μM H2O2 to energized WT mitochondria (n=3). (D) H2O2 levels of energized cortical and hippocampal mitochondria expressed as a ratio of relative fluorescent units (Fmax/Fmin) (n=3-5). (E) Left: Densimetric values of UCP2 protein immunofluorescent labeling normalized to VDAC in isolated mitochondria (n=6). Right: Functional uncoupling following application of free fatty acids (FA) to energized mitochondria in state IV was calculated as the percent increase in oxygen consumption (n=3-4). (F) Paired-pulse ratios (PPR) at baseline (Base, white) and following rotenone (Rot, green; n=6-7 slices, 6-7 mice). (G) Representative traces and quantification of extracellular CA3 SLE duration and Inter-SLE intervals in hippocampal slices following application of vehicle (Veh, white) or Rot (n=3-7 slices, 3-7 mice). Bars: 0.5mV × 5min. Data are expressed as the mean ± SEM, *p<0.05, **p<0.01, ***p<0.001.