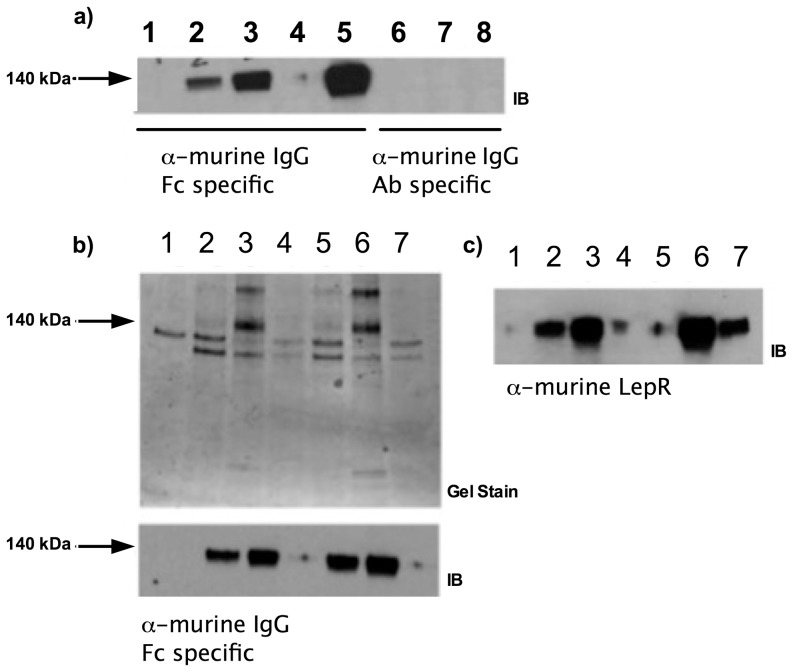
Figure 1. Expression and isolation of recombinant murine LepRec-Fc fusion proteins.
a) Expression of murine LepRec-Fc constructs in adherent HEK293T/17 cells. Supernatants containing LepRec-Fc chimera proteins from 24 and 48 hours post-transfection were cleared of cellular debris, subjected to SDS PAGE and western blotted with antibodies against domains of murine IgG1 (Fc specific for lanes 1–5 and Ab specific for lanes 6–8). Lane 1: Mock transfected at 48 hours. Lane 2: WT transfected at 24 hours. Lane 3: WT transfected at 48 hours. Lane 4: Q223R transfected at 24 hours. Lane 5: Q223R transfected at 48 hours. Lane 6: Mock transfected at 48 hours. Lane 7: WT transfected at 48 hours. Lane 8: Q223R transfected at 48 hours. b) Concentration and buffer exchange of murine LepRec-Fc chimeras. Supernatants were collected 48 hours after growth medium was replaced with expression medium (serum free Optimem +2 mM sodium butyrate). These supernatant preparations were concentrated by Amicon ultrafiltration (NMWCO of 100 kDa). Supernatants, concentrates, and filtrates from each chimera were subjected to SDS-PAGE followed by coomassie staining (top) and western blotting with α-murine IgG1 specific to the Fc region (bottom). Lane 1: Expression medium. Lane 2: WT supernatant. Lane 3: WT concentrate. Lane 4: WT filtrate. Lane 5: Q223R supernatant. Lane 6: Q223R Concentrate. Lane 7: Q223R Filtrate. c) Supernatants, concentrates, and filtrates (as in b) from each chimera were subjected to SDS-PAGE followed by transfer to PVDF membrane and western blotting with α-murine leptin receptor (R&D scientific). Lane 1: Expression medium. Lane 2: WT supernatant. Lane 3: WT concentrate. Lane 4: WT filtrate. Lane 5: Q223R Filtrate. Lane 6: Q223R Concentrate. Lane 7: Q223R supernatant.