Abstract
Previous clinical and experimental studies have indicated that cells responsible for IgA nephropathy (IgAN), at least in part, are localized in bone marrow (BM). Indeed, we have demonstrated that murine IgAN can be experimentally reconstituted by bone marrow transplantation (BMT) from IgAN prone mice in not only normal mice, but also in alymphoplasia mice (aly/aly) independent of IgA+ cells homing to mucosa or secondary lymphoid tissues. The objective of the present study was to further assess whether secondary lymph nodes (LN) contribute to the progression of this disease. BM cells from the several lines of IgAN prone mice were transplanted into aly/aly and wild-type mice (B6). Although the transplanted aly/aly showed the same degree of mesangial IgA and IgG deposition and the same serum elevation levels of IgA and IgA-IgG immune-complexes (IC) as B6, even in extent, the progression of glomerular injury was observed only in B6. This uncoupling in aly/aly was associated with a lack of CD4+ T cells and macrophage infiltration, although phlogogenic capacity to nephritogenic IC of renal resident cells was identical between both recipients. It is suggested that secondary LN may be required for the full progression of IgAN after nephritogenic IgA and IgA/IgG IC deposition.
Introduction
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis and exhibits mesangial IgA and IgG codeposition [1]. However, the mechanisms of mesangial IgA deposition and the origin of nephritogenic IgA remain unclear. Many studies have convincingly suggested the involvement of dysregulation in the mucosal immune system. Mesangial IgA and an increased serum IgA fraction in patients with IgAN are predominantly polymeric IgA1 (pIgA1) [2], [3]. Several studies have shown the numbers of IgA1+ plasma cells are increased in the bone marrow (BM) of patients with IgAN [4], [5]. Moreover, bone marrow transplantation (BMT) or peripheral blood stem cell transplantation in patients with leukemia and IgAN has resulted in a remission of leukemia as well as IgAN [6], [7]. These findings suggest that the cells responsible for producing pathogenic IgA1 may exist, at least in part, in the BM of IgAN patients.
The ddY mouse is known as a spontaneous IgAN prone mouse [8], although the incidence of their IgAN is highly variable [8], [9]. We found that the mice could be divided into the following three groups through a longitudinal histological analysis: early onset, late onset, and a quiescent group [10]. A genome-wide association study between the early onset and quiescent mice showed that one of the susceptibility loci of murine IgAN is syntenic to the susceptibility loci of human IgAN [10]–[12]. These findings indicated that this murine IgAN might be, at least in part, under the same genetic regulation as in human IgAN. Moreover, IgAN onset ddY mice exhibited elevated levels of serum IgA-containing immune-complexes (IC) and mesangial IgA and IgG co-deposition, as observed in human IgAN [13]. Nasal challenge with unmethylated CpG dinucleotides (CpG DNA), by which bacteria and viruses are distinguished and the Toll-like receptor (TLR)-9 is activated, worsened glomerular injury in the onset ddY mice and was associated with greater mesangial IgA deposition, higher serum IgA levels, and strong Th1 polarization [14]. We succeeded in generating several lines of a “grouped ddY mouse” that are a mouse model of IgAN with 100% onset after crossbreeding early onset mice for more than 20 generations [15]. Thus, it is suggested that the grouped ddY mouse can be a useful model for studying the pathogenic mechanisms of IgAN.
We also reported that BMT from the onset IgAN prone mice induced IgAN and that the serum levels of IgA-IgG IC were significantly correlated with the severity of glomerular injury [13], [16], [17]. However, the underlying mechanism by which the BM cells (BMC) induce IgAN remains unclear. Moreover, it was still unclear whether BMC directly produce nephritogenic IgA or require additional encounters with certain antigens in lymphoid tissues. To answer this question, we performed BMT and the adoptive transfer of cells from Peyer’s patches (PP) from IgAN prone mice and alymphoplasia mice (aly/aly) lacking PP, all lymph nodes (LN), and immunoglobulin producing cells due to a point mutation in the NF-κB-inducing kinase (NIK) gene on a C57BL/6 (B6) background [18]. A recent study demonstrated that the injection of PP cells of control mice into aly/aly mice induced both the migration of PP cells into the lamina propria (LP) and the generation of IgA+ plasma cells, thus rescuing gut IgA. On the other hand, the transplantation of BMC from normal control mice into aly/aly mice failed to rescue gut IgA, in spite of a recovery of serum IgA and the presence of IgA+ B cells and plasma cells [19]. Indeed, we observed that BMC induced glomerular IgA deposition independently of homing to the mucosa and secondary lymphoid tissues in the murine IgAN. Furthermore, BM may be a major reservoir of cells producing glomerular IgA. However, BMC could not induce the full progression of glomerular injury after IgA deposition in aly/aly mice. The objective of the present study using aly/aly mice was to further assess how secondary LN contribute to the progression of murine IgAN.
Materials and Methods
Ethics Statement
All animal studies were approved by the Ethics Review Committee for Animal Experimentation of the Juntendo University Faculty of Medicine. Animal procedures were conducted in compliance with National Institutes of Health Guidelines.
Mice
Two lines (A and B) of grouped ddY mice [10], [15], [17], aly/NSCJcl-aly (aly/aly) mice (CLEA Japan, Tokyo, Japan) with B6 background, and wild type C57/BL6 (B6) mice (SLC Japan) were maintained in the animal facility of Juntendo University in a specific-pathogen-free (SPF) room. For BMT, all mice of both lines of grouped ddY mice showed the disease phenotypes of IgAN from 4–6 weeks of age.
Bone Marrow Transplantation (BMT)
We analyzed the histological changes of the kidneys and measured the levels of serum immunoglobulins. The procedure for murine BMT was previously described in detail [13]. In brief, BMC were harvested from the tibia, femur and humerus of the IgAN onset ddY mice (A and B) [15] at 18–20 weeks of age under sterile conditions. Red blood cells were depleted from the collected BMC. Female aly/aly mice at 8–9 weeks of age and the same-aged B6 mice were used as recipients. Then, 1×107 BMC were injected into the tail vein of irradiated recipient mice at 700 rad. Transplanted mice were housed under SPF conditions. Transplanted aly/aly and control B6 mice were sacrificed at 12 and 24 weeks after BMT. We examined the histology of the kidneys and conducted a FACS analysis of spleen cells and BMC. The levels of serum immunoglobulins, IC, and urinary albumin were measured.
Histological Examination
For light microscopy, the kidney specimens were fixed in 20% neutral phosphate-buffered formalin, embedded in paraffin and then stained with periodic acid-Schiff (PAS) reagents. All specimens of BM transplanted mice were analyzed quantitatively to determine the percentages of glomeruli with (1) segmental and global sclerosis, (2) mesangial cell proliferation and cellular infiltration, and/or (3) an increase in the mesangial matrix. All specimens were scored semiquantitatively for the percentage of the aforementioned lesioned glomeruli (score 0, 0%; score 1, 1 to 19%; score 2, 20 to 39%; score 3, 40 to 59%; score 4, 60 to 79%; and score 5, >80% of all glomeruli) [20]. Glomerular polymorphonuclear cells (PMNs) of the anti-glomerular basement membrane (GBM) glomerulonephritis (GN) mice were counted in 3 µm sections that had undergone a PAS staining. The numbers of PMNs were determined in at least 20 glomeruli per mouse. For immunohistochemistry, the tissue samples were frozen in an OCT compound. Sections 5 µm thick were fixed in 4% paraformaldehyde. The following reagents were used for staining: FITC- rat anti-mouse IgA, FITC- rat anti-mouse IgG, FITC- rat anti-CD4 (BD Biosciences, Pharmingen; San Diego, CA), FITC- anti-mouse macrophages (F4/80) (Funakoshi, Tokyo, Japan), and rhodamine-conjugated goat anti-mouse IgA (Santa Cruz Biotechnology, Santa Cruz, CA) antisera. For electron microscopy, the specimens were fixed in phosphate-buffered glutaraldehyde for 2 hours, postfixed in 2% osmium tetroxide for 2 hr, and then embedded in Epon resin after dehydration. Ultrathin sections were sliced at 70 nm, stained with 4% uranyl acetate and lead citrate, and then examined under an electron microscope (Hitachi 7100, Tokyo, Japan).
Serum and Urinary Examination
Blood samples were obtained from the orbital venous plexus using capillary tubes. The serum samples were stored at −80°C prior to use. The serum levels of IgG and IgA were measured by single radioimmunodiffusion (SRL, Tokyo, Japan). Level of serum IgA/IgG IC was measured by sandwich ELISA according to a method described previously [13]. In brief, an ELISA plate was coated with 5 µg/ml rat anti-mouse-IgG antisera (Pharmingen) at 4°C overnight. Coated plates were blocked with 2% BSA in PBS, and serum samples were incubated at 4°C overnight. After incubation with HRP-conjugated goat anti-mouse IgA antisera (ZYMED Laboratories, San Francisco, CA) at room temperature for 3 hr, the reaction was developed with a tetramethylbenzidine substrate reagent (BD Biosciences, San Jose, CA), and the absorbance was measured at 540 nm. Data were expressed relative to the value of BALB/c mice. Urinary albumin was measured using an ELISA kit (Albuwell; Exocell, Philadelphia, PA). Serum and urine samples from BALB/c and B6 mice were used as controls.
Monocyte Chemotactic Protein (MCP)-1 Production in Primary Mesangial Cells
Mouse mesangial cells (MC) were cultured from outgrowths of glomeruli harvested as described previously [21]. In brief, aly/aly and control B6 mice were sacrificed by decapitation, and their kidneys were removed. After removal from the renal capsule, the kidneys were minced and treated with collagenase. The components of glomeruli were separated by repeated 30–50% Percoll gradient centrifugation and were cultured in an RPMI-1640 medium supplemented with 20% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, insulin, and transferine in a 5% CO2 environment at 37°C. On the 21st day of culture, MC were trypsinized and subcultured for 3 days in 6-well plates.
Heat-aggregated IgA was generated through the incubation of mouse myeloma IgA (Cappel, MP Biomedicals LLC., Solon, OH) at 63°C for 150 min [22]. After centrifugation, insoluble aggregates were discarded. After washing 3 times with a serum-free culture medium, subconfluent MC in 6-well plates were incubated with 200 µg/ml of heat-aggregated IgA in a 1 ml RPMI-1640 medium with 0.5% FCS. Cell-free supernatants were collected after 6, 12, and 24 hr of incubation and stored at −80°C until assayed for MCP-1 (n = 3, each). MCP-1 concentrations in the supernatants were measured using an OptEIA Mouse MCP-1 Set (Pharmingen).
Anti-glomerular Basement Membrane (GBM) Glomerulonephritis (GN) Induction
The method for preparation of nephrotoxic serum (NTS) was previously described [23]. In brief, NTS was kindly provided by Kyowa Hakko Kogyo Co. Ltd. (Mishima, Japan). Anti-GBM GN was induced by an intravenous injection of NTS through the tail vain in aly/aly mice (n = 3) and control B6 mice (n = 3). We collected the urine of the mice and sacrificed them 6 hours after the NTS injection. We examined the degree of polymorphonuclear cells (PMN) influx and the histology of the kidneys.
Statistical Analysis
Data were analyzed by using the paired Student’s t test. P values of less than 0.05 were considered as significant. All statistical analyses were performed using StatView 5.0 software (Abacus Concepts Inc., Berkeley, CA).
Results
Reconstitution of Glomerular IgA Deposition by BMC from the IgAN Prone Mice
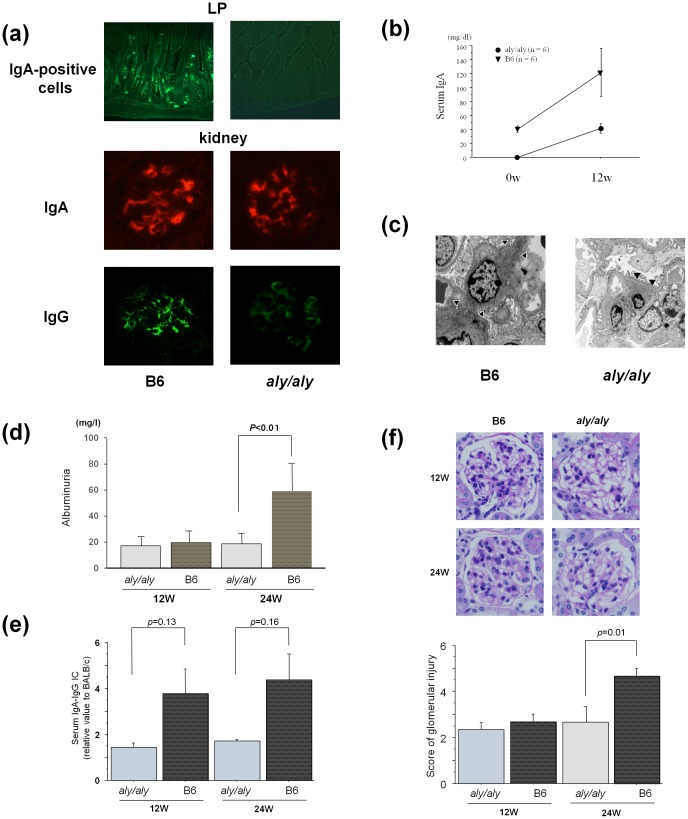
The BMT from IgAN onset grouped ddY mice (line A) reconstituted glomerular IgA deposition (Figure 1a), with an increase of serum IgA (Figure 1b) and the number of IgA+B220−CD138+ plasma cells (data not shown), in aly/aly mice at 12 weeks as well as in B6 mice (n = 6, each). However, the migration of IgA positive cells to the lamina propria was not observed in aly/aly mice at 12 weeks after BMT (Figure 1a). Paramesangial electron-dense deposits were confirmed by electron microscopy in the transplanted aly/aly mice (Figure 1c). These data suggest that BMC can directly reconstitute glomerular IgA deposition and the homing process in the LP is not required as reported in our previous study [24].
Figure 1. Reconstitution of IgA nephropathy by BM transplantation from ddY mice in aly/aly and B6 mice.
BM transplantation (BMT) from IgAN onset ddY mice (line A) (N = 24) reconstituted glomerular IgA (a) with serum elevation of IgA (b) in aly/aly and WT control B6 mice (aly/aly and B6 at 12 and 24 w, n = 6, each) independently of homing of IgA-positive cells into lamina propria (LP). Clear glomerular IgG deposition was found in B6, but not in aly/aly mice. Although electron-dense deposits (arrow head) were detected in aly/aly mice at 24 w after BMT as well as B6 mice (c), progression of urinary protein (d) and glomerular lesions (f) were absent in aly/aly mice in association with the lack of serum elevation of IgA-IgG immune complex (IC) (e). Data are presented as mean ± SD.
Uncoupling of Glomerular IgA Deposition and Disease Progression in Transplanted Aly/Aly Mice from IgAN Prone Mice (Line A)
Reconstituted glomerular IgA deposition, the level of albuminuria, and the glomerular injury score were not significantly different between aly/aly and B6 recipients at 12 weeks after the BMT (Figure 1d). The progression of albuminuria and glomerular injury with an increase of IgA-IgG IC (Figure 1e), mesangial cell proliferation, and mesangial matrix expansion were observed in the B6 recipient mice after 24 weeks (Figures 1f). On the other hand, although the transplanted aly/aly mice showed glomerular IgA deposition, they did not show either an increase of IgA-IgG IC and clear glomerular injury or a progression of albuminuria at 24 week after BMT (Figures 1a, c–f).
No Difference of Phlogogenic Capacity to Nephritogenic Antibody of Renal Resident Cells in both Aly/Aly and B6 Mice
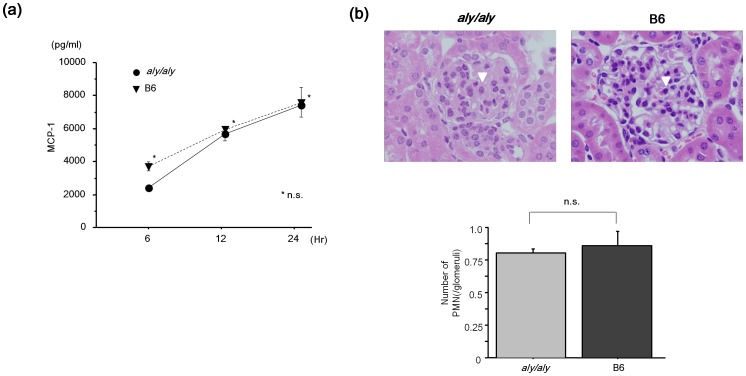
To approach underlying mechanism for the uncoupling of IgA deposition and progression of glomerular injury in aly/aly mice, we first checked the phlogogenic capacity to nephritogenic antibody of renal resident cells in both mice with or without the NIK mutation. MC-derived chemokine, MCP-1, mainly depending on NF-κB activation, is known as a key mediator for the progression of IC-induced glomerulonephritis (24). The primary MC from aly/aly and B6 mice was stimulated by heat-aggregated IgA and the MCP-1 production was evaluated, in order to exclude the possibility that an NIK mutation in the intrinsic renal cells of aly/aly mice may directly influence in vivo findings. MCP-1 production was increased in a time-dependent manner in both aly/aly and B6 mice (Figure 2a). In addition, these levels were not significantly different in both mice, suggesting that major chemokine production by MC after IgA deposition may be independent of the NIK mutation in aly/aly mice.
Figure 2. Identical phlogogenic capacity of renal resident cells in aly/aly and B6 mice.
(a) To confirm the nephritogenic capacity to of renal resident cells in aly/aly and B6 mice, chemokine expressions, such as MCP-1, were examined with mesangial cells from both mice under stimulation with IgA IC. MCP-1 concentrations in supernatants of both mice were not significantly different (n = 3, each). (b) Next to confirm phlogonenic capacity of aly/aly mice, anti-GBM glomerulonephritis was induced in aly/aly and B6 mice (n = 3 each). An influx of PMN (arrow head) was shown in the glomerulus of both mice. The number of PMN in the glomerulus of aly/aly and B6 mice were not significantly different. Data are presented as mean ± SD.
No Difference of the Response of Anti-GBM GN in Aly/Aly Mice and B6 Mice
To further examine the phlogogenic capacity of aly/aly mice, we next used a different acute in vivo model. Anti-GBM GN was induced in aly/aly and B6 mice. In both mice, we observed a glomerular PMN influx, and the number of PMN in these glomeruli was not significantly different between aly/aly and B6 mice (Figure 2b). Thus, the acute phlogogenic capacity of aly/aly mice is not different from B6 mice.
The Uncoupling of Glomerular IgA Deposition and Disease Progression in Transplanted Aly/Aly Mice from the IgAN Prone Mice
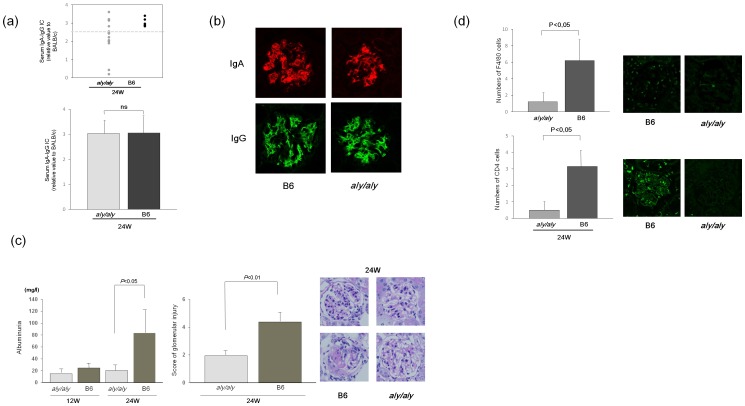
In BMT from line A, IgG co-deposition at 24 weeks of age in aly/aly mice was less than that of B6 mice in association with lower levels of serum IgA-IgG IC (Figure 1). To assess whether lower IgG IC formation and its glomerular deposition may result in an uncoupling in aly/aly mice, we transplanted BM from grouped ddY mice line B, which show poorer prognosis in association with higher IC formation than line A [15]. Serum levels of IgA-IgG IC formation at 24 weeks in aly/aly mice transplanted from line B were variable but higher than those from line A (Figure 1e and 2a). Although they were lower than those in the control B6 on average, some of aly/aly recipients from line B (more than 2.5; N = 5) (Figure 3a) showed similar levels of serum IgA-IgG IC and IgG co-deposition as the control (Figure 3b). However, even in the selected aly/aly mice (n = 5) that showed similar amounts of serum IgA-IgG IC and IgG codepositions as the B6 control (n = 5), the progression of renal damage, including albuminuria and an injury score, was absent (Figure 3c) as compared to BMT from line A. This is associated with the lack of F4/80 (macrophages) and CD4+ T cells infiltration (Figure 3d).
Figure 3. Uncoupling of glomerular IgA and disease progression in transplanted aly/aly mice was independent of IgA-IgG IC formation and deposition.
To further assess underlying mechanisms in the lack of the disease progression of IgAN in aly/aly mice, BM cells from a different line of poor-prognosis IgAN prone (line B) were transplanted in both mice. Although the serum levels of IgA-IgG IC were variable, some of the transplanted aly/aly mice (n = 5) showed similar amounts of IC (>2.5) to B6 mice (n = 5) (a). These selected aly/aly mice showed clear glomerular deposition of not only IgA but also IgG at 24 w, as seen in B6 mice (b). However, the progression of proteinuria and glomerular lesions was absent (c). This was associated with lack of F4/80 positive macrophages and CD4+ T cells (d). Data are presented as mean ± SD.
Discussion
Several studies have shown that the number of IgA1+ plasma cells increased in the BM of patients with IgAN [4], [5] and that BMT or peripheral blood stem cell transplantation in patients with leukemia and IgAN resulted in a remission of the leukemia as well as the IgAN [6], [7], suggesting that the producing cells of nephritogenic IgA may be in the BM of IgAN. Indeed, we reported that BMT from onset IgAN-prone mice induced IgAN in healthy mice [13]. However, it is clinically well known that many IgAN patients show episodic macrohematuria coincident with mucosal infection, especially in the upper respiratory tract [26]–[28]. Mucosal vaccination studies revealed abnormal immune responses in the mucosa of patients with IgAN [29], [30]. Importantly, there is increasing evidence that tonsillectomy has a favorable effect on the long-term renal survival of IgAN patients [31]–[33]. We reported that TLR9 activation by nasal challenge with CpG DNA worsened glomerular injury in ddY mice and was associated with strong Th1 polarization [14], [34], [35]. In this regard, several reports support the idea that exogenous antigens via the respiratory tract or gut induce glomerular IgA deposition [14, 34, 36–38]. Therefore, these observations indicate that the mucosal immune response of IgAN patients might play a role in pathogenesis, and furthermore, responsible cells may be disseminated to not only the BM, but also to mucosa or systemic lymphoid tissues [16].
Our previous study demonstrated that BMC of onset ddY mice could reconstitute glomerular IgA deposition in aly/aly mice lacking PP, all LN and without migration of IgA+ cells to LP [24]. These findings suggested that the mechanism of nephritogenic IgA production might not require other immune responses or the maturation of BMC in secondary lymphoid tissues and mucosa, at least in murine IgAN. In addition, we first showed that the homing process of IgA+ cells into the mucosa is not necessary for nephritogenic IgA production. On the other hand, PP transfer of onset ddY mice could not reconstitute glomerular IgA deposition [24]. The findings suggest that producing cells of nephritogenic IgA do not constitute PP of onset ddY mice. Accordingly, we hypothesized that the mucosa may be the major initial priming site of cells responsible for nephritogenic IgA production, while BM may be a major reservoir of these cells, presumably memory type cells, and a major site of nephritogenic IgA production [39].
This raises the question as to whether an NIK mutation in intrinsic renal cells of aly/aly mice may protect renal injuries followed by mesangial IgA deposition. Several studies [25], [40] have clearly demonstrated that the activation of NF-κB, known as a key inflammatory transcriptional factor in renal resident cells, is a major process in the progression of IC-mediated glomerulonephritis. MCP-1 has an important role in the development of renal injuries [41], [42]. Emerging evidence has indicated that the upregulation of glomerular MCP-1 during the proliferative phase of glomerulonephritis is, at least in part, due to the NF-κB dependent signaling pathway [43], [44]. Levels of MCP-1 produced by mesangial cells stimulated with heat-aggregated IgA in aly/aly mice did not differ from those in B6 mice. It is suggested that the NIK mutation, at least in mesangial cells, may not affect major chemokine production by MC and the progression of IgAN, such as albuminuria, proliferation of mesangial cells, and the expansion of mesangial matrix, followed by mesangial IgA deposition in transplanted aly/aly mice. To further confirm this idea, we checked the phlogogenic capacity of aly/aly mice in a different in vivo model. The anti-GBM GN model is often used to investigate acute inflammatory cascade leading to immunologically-mediated GN [45]. Anti-GBM GN consists of two distinct phases, namely a heterologous phase, resulting from a rapid binding of the injected heterologous anti-GBM antibodies, and autologous phase that is induced when the recipient’s cells react with its own antibodies against the heterologous immunoglobulin previously fixed at the GBM. The severity of renal damage in the heterologus phase is known to be dependent on the glomerular influx of PMN [21], [45]. Our study shows that a normal influx of PMNs as heterologus phase of the anti-GBM GN is also induced in aly/aly and B6 mice. Although we may need to further analyze the effect of the NIK mutation in kidney disease, these results show that the NIK mutation is not likely to have an impact in the absence of an influx of PMN in the acute immune response after antibody deposition and glomerular injuries, representative of a proliferation of mesangial cells. In addition, previous studies showed that present protocol of BMT functionally exchanged most of leukocytes in recipients from original to transferred BM-derived leukocytes [14], [15], [46], [47]. Therefore, the uncoupling of glomerular IgA deposition and disease progression in the transplanted aly/aly mice from onset ddY mice is not influenced by the NIK mutation.
In general, the mild glomerular deposition of IgA is not always accompanied by proteinuria and hematuria. Moreover, in so-called secondary IgAN [48], [49] observed in patients with liver cirrhosis [50], portal systemic shunts [51], dermatitis herpetiformis [52], celiac disease [53], and chronic inflammatory disease of the lung [54], glomerular IgA deposition without obvious glomerular lesions [48] is frequently observed. Meanwhile, many studies have demonstrated emerging evidence that mesangial IgA in IgAN is exclusively the aberrantly glycosylated IgA1 subclass, in particular, galactose-deficient in the hinge region of IgA1 (GdIgA) [55], [56]. However, Gharavi et. al. reported that a high serum level of GdIgA1 was determined in a cohort of not only IgAN patients but also their relatives without nephropathy [57]. This finding indicates that other mechanisms besides GdIgA1 are required for the full progression of IgAN.
Although the functional significance of the altered IgA is still obscure, Tomana et al. reported that the level of circulatory autoantibodies against aberrantly glycosylated IgA1 was elevated in patients with IgAN [58]. Recently, Suzuki et al. reported that the development of a dot-blot assay using Gal-deficient IgA1 as an antigen and GalNAc-specific recombinant human IgG from an IgAN patient as a standard permitted accurate analysis of the levels of glycan-specific IgG in sera with high specificity and sensitivity and the serum levels of this IgG, which is specific for Gal-deficient IgA1, correlated with the clinical parameter of proteinuria [59]. It suggests that aberrantly glycosylated IgA1 is recognized as an endogeneous antigen and forms IC with circulatory autoantibodies in human IgAN [59]. Such multi-hit processes are now hypothesized in IgAN [60] and may decide the heterogeneity of IgAN [61].
This picture is also seen in murine IgAN. The severity of murine IgAN is linked to to the serum levels of IgA-IgG IC but not IgA [14]. On the other hand, the progression of murine IgAN may also link to abberant glycosylation of IgA as seen human IgAN [15]. We recently reported that polymerization or IC formation, presumably due to aberrantly glycosylated IgA, is important for the complement activation in murine IgAN [17]. Furthermore, mice lacking β-1,4-galactosyltransferase-I, which transfers galactose to the terminal N-acetylglucosamine of N-linked glycans in a β-1,4 linkage, showed a complete absence of β4 galactosylation on the N-glycans of mouse IgA and subsequently spontaneous development of IgAN with increased serum polymeric IgA levels [62]. These findings suggest that the aberrant glycosylation of serum IgA, presumably as an endogeneous antigen, may be involved in the induction of IgAN, even in mice. Therefore, we initially speculated that the transplanted aly/aly mice from line A could not form IC containing antibodies recognized as an endogeneous antigen because the mice are lacking secondary lymphoid tissues and because the mice did not induce sufficient IgA/IgG co-deposition and glomerular injury.
To confirm this idea, we further did BMT with different clones of grouped ddY mice line B, which we recently established by cross-breeding [15]. This prone mouse shows a more severe prognosis of murine IgAN with an increase of serum levels of IgA-IgG IC [13], [17] that may be partly due to the pattern of glycosylation in IgA. In this additional model, we indeed found a greater increase of IgA-IgG IC and clear IgG deposition. This finding suggests that secondary lymphoid tissues may be not required for at least the circulating IC formation. However, even though we selected aly/aly recipients showing similar amounts of glomerular IgG co-deposition, full progression of murine IgAN in aly/aly recipients was absent, suggesting other possible underlying mechanisms. Interestingly, this uncoupling in aly/aly mice is linked to a lack of CD4+ T cells and macrophage infiltration. It is recently known that the renal prognosis of IC-mediated glomerulonephritis, including IgAN and lupus nephritis, is significantly associated with the degree of T cell infiltration [63]–[65]. Accordingly, antigen presentation in the renal LN and the subsequent activation of cellular immunity may be involved in chronic and full progression of murine IgAN after the nephritogenic IgA and IC deposition in glomerular mesangial areas.
The uncoupling of glomerular IgA/IC deposition and disease progression associated with a lack of CD4 and macrophage infiltration in aly/aly mice indicates that secondary LN, presumably renal LN, may be required for the activation of cellular immunity and chronic progression of this disease. Multi-hit theory in the pathogenesis of IgAN [60] should involve the mechanisms in renal LN after the deposition of nephritogenic IgA/IC.
Acknowledgments
We thank T. Shibata, and all members of the laboratory for their technical support and discussions.
Funding Statement
A part of this study was supported by research funds from Grant-in-Aid for Progressive Renal Disease Research, Research on Intractable Disease, from the Ministry of Health, Labor and Welfare of Japan and a grant from the Study Group on IgA Nephropathy in Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Burger J, Hinglais N (1968) Les Depots intercapillaries d’IgA-IgG. Urol Nephrol Paris 74: 694–695. [PubMed] [Google Scholar]
- 2. Tomino Y, Endoh M, Nomoto Y, Sakai H (1981) Immunoglobulin A1 and IgA nephropathy. N Engl J Med. 305: 1159–1160. [DOI] [PubMed] [Google Scholar]
- 3. Barratt J, Feehally J (2005) IgA nephropathy. J Am Soc Nephrol 16: 2088–2097. [DOI] [PubMed] [Google Scholar]
- 4. van den Wall Bake AW, Daha MR, Evers-Schouten J, van Es LA (1988) Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis 12: 410–414. [DOI] [PubMed] [Google Scholar]
- 5. Harper SJ, Allen AC, Pringle JH, Feehally J (1996) Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridisation for J chain mRNA. J Clin Pathol 49: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakai O (1997) IgA nephropathy: current concepts and future trends. Nephrology 3: 2–3. [Google Scholar]
- 7. Iwata Y, Wada T, Uchiyama A, Miwa A, Nakaya I, et al. (2006) Remission of IgA nephropathy after allogeneic peripheral blood stem cell transplantation followed by immunosuppression for acute lymphocytic leukemia. Intern Med 45: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 8. Imai H, Nakamoto Y, Asakura K, Miki K, Yasuda T, et al. (1985) Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int. 27: 756–761. [DOI] [PubMed] [Google Scholar]
- 9. Shimizu M, Tomino Y, Abe M, Shirai T, Koide H (1992) Retroviral envelope glycoprotein (gp 70) is not a prerequisite for pathogenesis of primary immunoglobulin A nephropathy in ddY mice. Nephron. 62: 328–331. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Nishimura H, et al. (2005) Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10, in a region syntenic to human IGAN1 on chromosome 6q22–23. J Am Soc Nephrol 16: 1289–1299. [DOI] [PubMed] [Google Scholar]
- 11. Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, et al. (2000) IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22–23. Nat Genet. 26: 354–357. [DOI] [PubMed] [Google Scholar]
- 12.Takei T, Iida A, Nitta K, Tanaka T, Ohnishi Y, et al. (2002) Association between single-nucleotide polymorphisms in selectin genes and immunoglobulin A nephropathy. Am J Hum Genet 70: : 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, et al. (2007) Th1 polarization in murine IgA nephropathy directed by bone marrow-derived cells. Kidney Int. 72: 319–327. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, et al. (2008) Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 19: 2384–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okazaki K, Suzuki Y, Otsuji M, Suzuki H, Kihara M, et al. (2012) Development of a model of early-onset IgA nephropathy. J Am Soc Nephrol. 23: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, et al. (2013) Experimental evidence of cell dissemination playing a role in pathogenesis of IgA nephropathy in multiple lymphoid organs. Nephrol Dial Transplant. 28: 320–326. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto A, Suzuki Y, Suzuki H, Ohsawa I, Brown R, et al. (2012) Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes. Am J Pathol. 181: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, et al. (1999) Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet 22: 74–77. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki K, Meek B, Doi Y, Honjo T, Fagarasan S (2005) Two distinctive pathways for recruitment of naive and primed IgM+ B cells to the gut lamina propria. Proc Natl Acad Sci USA 102: 2482–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katafuchi R, Kiyoshi Y, Oh Y (1998) Glomerular score as a prognosticator in IgA nephropathy: Its usefulness and limitation. Clin Nephrol 1: 1–8. [PubMed] [Google Scholar]
- 21. Kanamaru Y, Nakao A, Tanaka Y, Inagaki Y, Ushio H, et al. (2003) Involvement of p300 in TGF-beta/Smad-pathway-mediated alpha2(I) collagen expression in mouse mesangial cells. Nephron Exp Nephrol. 95: e36–42. [DOI] [PubMed] [Google Scholar]
- 22. Tsuge T, Suzuki Y, Shimokawa T, Horikoshi S, Okumura K, et al. (2003) Monocyte chemoattractant protein (MCP)-1 production via functionally reconstituted Fcalpha receptor (CD89) on glomerular mesangial cells. Inflamm Res. 52: 428–432. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki Y, Shirato I, Okumura K, Ravetch JV, Takai T, et al. (1998) Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 54: 1166–1174. [DOI] [PubMed] [Google Scholar]
- 24. Aizawa M, Suzuki Y, Suzuki H, Pang H, Kihara M, et al. (2007) Roles of bone marrow, mucosa and lymphoid tissues in pathogenesis of murine IgA nephropathy. Contrib Nephrol (157) 164–168. [DOI] [PubMed] [Google Scholar]
- 25. López-Franco O, Suzuki Y, Sanjuán G, Blanco J, Hernández-Vargas P, et al. (2002) Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol 161: 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholls KM, Fairley KF, Dowling JP, Kincaid-Smith P, (1984) The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 53: 227–250, 1984. [PubMed]
- 27. Feehally J, Beattie TJ, Brenchley PE, Coupes BM, Mallick NP, et al. (1986) Sequential study of the IgA system in relapsing IgA nephropathy. Kidney Int. 30: 924–931. [DOI] [PubMed] [Google Scholar]
- 28. Emancipator SN (1990) Immunoregulatory factors in the pathogenesis of IgA nephropathy. Kidney Int. 38: 1216–1229. [DOI] [PubMed] [Google Scholar]
- 29. de Fijter JW, Eijgenraam JW, Braam CA, Holmgren J, Daha MR, et al. (1996) Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 50: 952–961. [DOI] [PubMed] [Google Scholar]
- 30. van den Wall Bake AW, Beyer WE, Evers-Schouten JH, Hermans J, Daha MR, et al. (1989) Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest. 84: 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie Y, Chen X, Nishi S, Narita I, Gejyo F (2004) Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 65: 1135–1144. [DOI] [PubMed] [Google Scholar]
- 32. Sato M, Hotta O, Tomioka S, Horigome I, Chiba S, et al. (2003) Cohort study of advanced IgA nephropathy: efficacy and limitations of corticosteroids with tonsillectomy. Nephron Clin Pract (93) c137–145. [DOI] [PubMed] [Google Scholar]
- 33. Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, et al. (2008) Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: a controlled study. Clin J Am Soc Nephrol. 3: 1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kajiyama T, Suzuki Y, Kihara M, Suzuki H, Horikoshi S, et al. (2011) Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol. 2011: 819646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato D, Suzuki Y, Kano T, Suzuki H, Matsuoka J, et al. (2012) Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant. 27: 1090–1097. [DOI] [PubMed] [Google Scholar]
- 36. Coppo R, Mazzucco G, Martina G, Roccatello D, et al. (1989) Gluten-induced experimental IgA glomerulopathy. Lab Invest. 60: 499–506. [PubMed] [Google Scholar]
- 37. Jessen RH, Emancipator SN, Jacobs GH, Nedrud JG (1992) Experimental IgA-IgG nephropathy induced by a viral respiratory pathogen. Dependence on antigen form and immune status. Lab Invest. 67: 379–386. [PubMed] [Google Scholar]
- 38. Pestka JJ, Moorman MA, Warner RL (1989) Dysregulation of IgA production and IgA nephropathy induced by the trichothecene vomitoxin. Food Chem Toxicol. 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 39. Suzuki Y, Tomino Y (2007) The mucosa-bone-marrow axis in IgA nephropathy. Contrib Nephrol. 157: 70–79. [DOI] [PubMed] [Google Scholar]
- 40. Gómez-Guerrero C, López-Franco O, Suzuki Y, Sanjuán G, Hernández-Vargas P, et al. (2002) Nitric oxide production in renal cells by immune complexes: Role of kinases and nuclear factor-kappaB. Kidney Int 62: 2022–2034. [DOI] [PubMed] [Google Scholar]
- 41. Stahl RA, Thaiss F, Disser M, Helmchen U, Hora K, Schlöndorff D (1993) Increased expression of monocyte chemoattractant protein-1 in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int. 44: 1036–1047. [DOI] [PubMed] [Google Scholar]
- 42. Saitoh A, Suzuki Y, Takeda M, Kubota K, Itoh K, et al. (1998) Urinary levels of monocyte chemoattractant protein (MCP)-1 and disease activity in patients with IgA nephropathy. J Clin Lab Anal. 12: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ozawa Y, Kobori H, Suzaki Y, Navar LG (2007) Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 292: F330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen YM, Hu-Tsai MI, Lin SL, Tsai TJ, Hsieh BS (2003) Expression of CX3CL1/fractalkine by mesangial cells in vitro and in acute anti-Thy1 glomerulonephritis in rats. Nephrol Dial Transplant. 18: 2505–2514. [DOI] [PubMed] [Google Scholar]
- 45.Wilson CB (1996) The Kidney (5th ed.): Renal response to immunologic glomerular injury. In: Brenner Bm editor. Philadelphia: WB Saunders 1253−1391.
- 46. Suzuki Y, Gómez-Guerrero C, Shirato I, López-Franco O, Hernández-Vargas P, et al. (2002) Susceptibility to T cell-mediated injury in immune complex disease is linked to local activation of renin-angiotensin system: the role of NF-AT pathway. J Immunol. 169: 4136–4146. [DOI] [PubMed] [Google Scholar]
- 47. Suzuki Y, Gómez-Guerrero C, Shirato I, López-Franco O, Gallego-Delgado J, et al. (2003) Pre-existing glomerular immune complexes induce polymorphonuclear cell recruitment through an Fc receptor-dependent respiratory burst: potential role in the perpetuation of immune nephritis. J Immunol. 170: 3243–3253. [DOI] [PubMed] [Google Scholar]
- 48. Varis J, Rantala I, Pasternack A, Oksa H, Jäntti M, et al. (1993) Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol 46: 670–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clarkson AR, Woodroffe AJ, Bannister KM, Odum J, Thomas A (1984) The syndrome of IgA nephropathy. Clin Nephrol 21: 7–14. [PubMed] [Google Scholar]
- 50. Nakamoto Y, Iida H, Kobayashi K, Dohi K, Kida H, et al. (1981) Hepatic glomerulonephritis. Characteristics of hepatic IgA glomerulonephritis as the major part. Virchows Arch A Pathol Anat Histol. 392: 45–54. [DOI] [PubMed] [Google Scholar]
- 51. Woodroffe AJ, Clarkson AR, Seymour AE, Lomax-Smith JD (1982) Mesangial IgA nephritis. Springer Semin Immunopathol. 5: 321–332. [DOI] [PubMed] [Google Scholar]
- 52. Moorthy AV, Zimmerman SW, Maxim PE (1978) Dermatitis herpetiformis and celiac disease: association with glomerulonephritis, hypocomplementia, and circulating immune complexes. JAMA 239: 2019–2020. [DOI] [PubMed] [Google Scholar]
- 53. Katz A, Dyck RF, Bear RA (1979) Celiac disease associated with immune complex glomerulonephritis. Clin Nephrol 11: 39–44. [PubMed] [Google Scholar]
- 54. Endo Y, Hara M (1986) Glomerular IgA deposition in pulmonary diseases. Kidney Int 29: 557–562. [DOI] [PubMed] [Google Scholar]
- 55. Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, et al. (2001) Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 59: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 56. Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, et al. (2001) Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 60: 969–973. [DOI] [PubMed] [Google Scholar]
- 57. Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, et al. (2008) Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 19: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, et al. (1999) Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 104: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suzuki H, Fan R, Zhang Z, Brown R, Hall S, et al. (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, et al. (2011) The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 22: 1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Glassock RJ (2009) Analyzing antibody activity in IgA nephropathy. J Clin Invest 119: 1450–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nishie T, Miyaishi O, Azuma H, Kameyama A, Naruse C, et al. (2007) Development of immunoglobulin A nephropathy- like disease in beta-1,4-galactosyltransferase-I-deficient mice. Am J Pathol 170: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van Es LA, de Heer E, Vleming LJ, van der Wal A, Mallat M, et al. (2008) GMP-17-positive T-lymphocytes in renal tubules predict progression in early stages of IgA nephropathy. Kidney Int. 73: 1426–1433. [DOI] [PubMed] [Google Scholar]
- 64. Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, et al. (2009) Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 20: 2518–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Couzi L, Merville P, Deminière C, Moreau JF, Combe C, et al. (2007) Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum. 56: 2362–2370. [DOI] [PubMed] [Google Scholar]