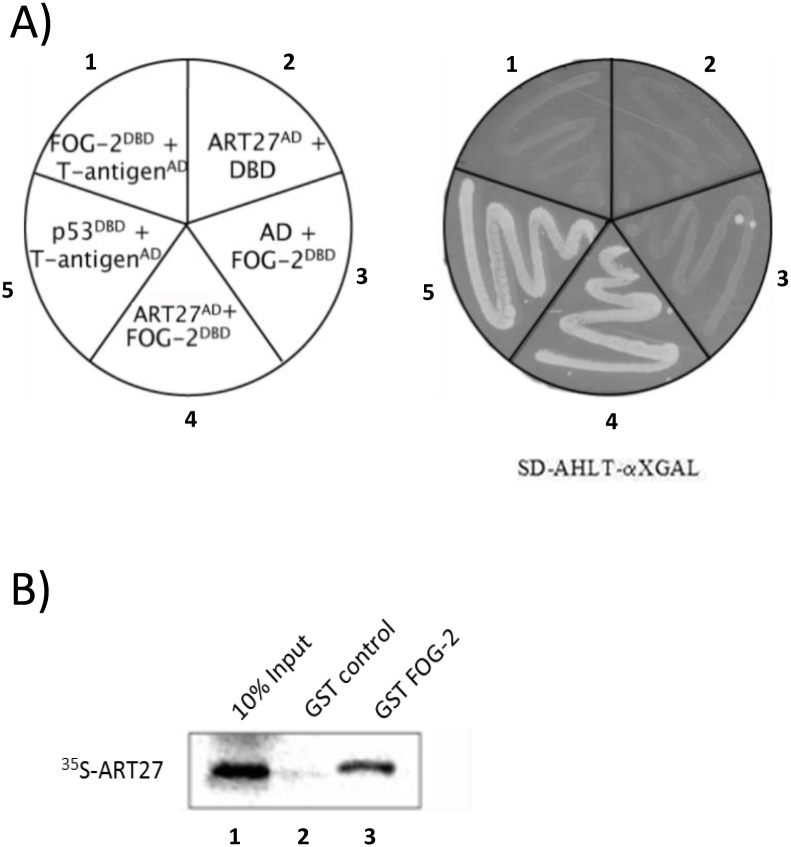
Figure 1. FOG-2 physically interacts with Art27.
(A) AH109 yeast were transformed with the respective bait and prey constructs and plated on synthetic dropout media lacking adenine, histidine, leucine and tryptophan and tested for X-GAL positive yeast growth. FOG-2 (amino acids 856–1156) failed to physically interact T-antigen (segment 1- negative control), Art27 and FOG-2 (856–1156) failed to autoactivate yeast growth (segment 2 and 3 respectively), Art27 and FOG-2 (856–1156) physically interact and promote yeast growth (segment 4) and as expected the physical interaction between p53 and T-antigen promoted yeast growth (segment 5 – positive control). (B) In vitro translated and 35S radiolabeled Art27 protein was incubated with full length FOG-2/GST fusion protein or GST that was immobilised on glutathione sepharose beads. After extensive washing the proteins were resolved by electrophoresis and detected using a phosphorimager. 35S labelled Art27 was retained only by the FOG-2/GST fusion protein (lane 3) indicating that they physically interact.