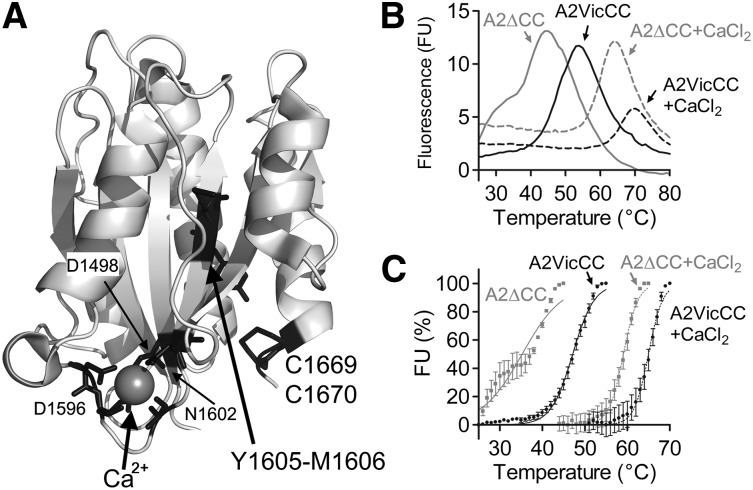
Figure 1.
Structure and thermodynamic unfolding of the VWF A2 domain. (A) Crystal structure of the VWF A2 domain (PDB 3ZQK, modified in Pymol) is shown with the vicinal disulphide bond (C1669, C1670), scissile bond (Y1605, M1606), and Ca2+-coordinating residues in black. (B) DSF measurements using the dye SyproOrange were taken at 510 nm every 1°C, with a ramp 1°C min−1, over a temperature range of 25°C to 80°C. FU reads were individually baseline corrected to a buffer only control with EDTA or CaCl2. Representative curves of the VWF A2 domain constructs A2VicCC and A2∆CC in the presence of an excess of 5 mM CaCl2 or 1mM EDTA to chelate-free Ca2+ are shown. (C) The unfolding curves were normalized, the lowest FU value was assigned as 0% and the highest FU value represents 100%. Resultant curves were fitted using the sigmoidal Boltzmann equation in Graphpad and the transition midpoint (Tm) calculated, see Table 1. Results are means of at least 3 independent experiments ± standard error of the mean (SEM).