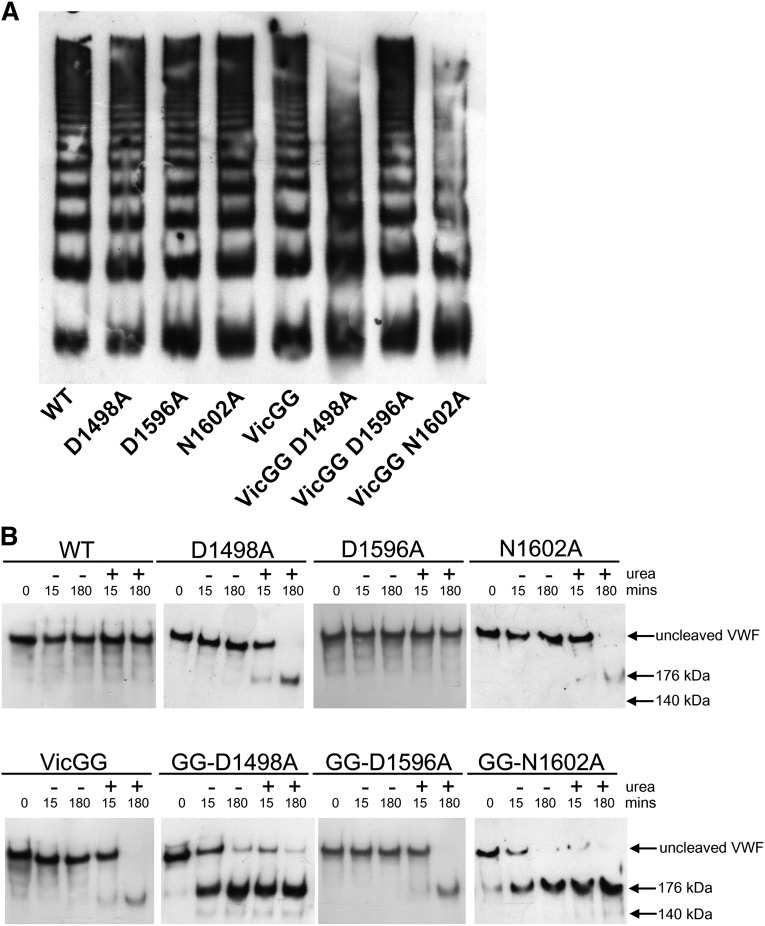
Figure 4.
Characterization and proteolysis of the vicinal cysteine and Ca2+ variants in FL-VWF. FL-VWF containing single-point mutations of the Ca2+-binding residues, alone or in combination with mutation of the vicinal cysteines to glycines (VWF-VicGG), were expressed in HEK293 cells. (A) Multimer formation was analyzed on a 1.4% agarose gel and VWF bands detected on western blot with anti-VWF polyclonal antibody to determine the multimer composition of the FL-VWF variants. (B) Proteolysis of FL-VWF by ADAMTS13. The VWF variants and ADAMTS13 were separately preincubated in 20 mM Tris (pH 7.8), 50 mM NaCl, 5 mM CaCl2 ± 1.5 M urea at 37°C for 45 minutes. VWF (1 µg/mL) and ADAMTS13 (5 nM) were then combined, incubated at 37°C and reactions stopped after 15 minutes and 180 minutes by the addition of EDTA. VWF cleavage products were resolved after reduction with 2-mercaptoethanol. The samples were run on a 3% to 8% Tris-acetate gel and VWF bands on western blot detected using anti-VWF antibody.