Abstract
Although primary chronic hypertension (HTN) is increasingly common in adolescence, secondary forms of HTN are more common among children. Primary HTN is associated with being overweight and/or a positive family history of HTN. Carotid intima-media thickness, a known risk factor for atherosclerosis is frequent in both adults and children with HTN and other associated cardiovascular (CV) risk factors including obesity, dyslipidemia, diabetes and chronic kidney disease. Left ventricular (LV) hypertrophy is also a common finding in children and adolescents with newly diagnosed HTN. Children with certain medical conditions such as congenital heart disease and Kawasaki disease can develop premature atherosclerosis heart disease that may lead to coronary heart disease and heart failure. Life-style interventions are recommended for all children with HTN, with pharmacologic therapy added for symptomatic children based on the presence of co-morbidities. As an example, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blocker and/or calcium channel blockers would be best for children with CV risk factors such as diabetes or renal disease, whereas an ACE inhibitor in combination with a beta-blocker and diuretics including spironolactone are recommended for patients with heart failure and reduced LV ejection fraction. This report will summarize new developments in the management of pediatric HTN complicated with CV disease and heart failure and will address the appropriate antihypertensive therapy that could potentially reduce the future burden of adult CV disease.
Keywords: Atherosclerosis, cardiovascular disease, heart failure, hypertension
INTRODUCTION
As in adults, children with hypertension (HTN) are at increased risk of cardiovascular (CV) events including left ventricular (LV) hypertrophy, increased carotid intima-media thickness, atherosclerosis, reduced arterial compliance and diastolic dysfunction.[1,2,3,4,5,6] Children and adolescents with certain medical conditions such as diabetes, microalbuminuria and elevated C-reactive protein,[6] familial hypercholestromia,[7] Kawasaki disease,[8] congenital heart disease,[9] chronic kidney disease (estimated glomerular filtration rate <60 mL/min)[10] experience accelerated arteriosclerosis that may lead to very early CV events and coronary heart disease, heart failure, cor pulmonale, pericardial effusions and arrhythmias. Early arteriosclerosis is seen in the majority of these diseases, with diabetes mellitus, chronic kidney disease and chronic inflammatory illnesses of particular note.[1,2,4] However, most prevalent of all the cardiac morbidities is systemic HTN. The reported prevalence of systemic HTN in children ranges from 1% to 4% with increasing prevalence of HTN paralleling the rise in childhood obesity.[11] Other major risk factors for CV events include cigarette smoking, obesity (body mass index ≥30 kg/m2), physical inactivity, dyslipidemia and family history of premature, CV disease and components of the metabolic syndrome.[1,2,3,4,5,6]
For children with diagnoses like these, intensive CV risk reduction is of critical importance.[12] Presence of metabolic syndrome can increase a person's risk quickly.[11,12] As an example, when Stage 1 HTN added to borderline high total cholesterol nearly triples a patient's 10 year CV disease risk compared with total cholesterol of 200 mg/dL alone.[13] Diabetes plus low-high-density lipoprotein cholesterol increases a patient's 10 year CV disease risk 5 times higher than it would be with only one risk factor. The Framingham risk score (age, total cholesterol, systolic blood pressure [BP], tobacco smoking) is the cornerstone for risk stratification of asymptomatic individuals and helps determine the intensity of therapy.
MANAGEMENT OF HYPERTENSIVE PATIENTS WITH CV DISEASE
Current guidelines for the management of HTN in children and adolescents following CV events recommend pharmacological therapy should be initiated with stage 2 HTN[2] Antihypertensive medications should begin with the recommended initial dose of desired medication and should be titrated upward until the BP target is reached. Consider adding a second medication with a complementary mechanism of action if BP control is not achieved. A combination of a beta-blocker and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) in low doses is the preferred choice. Add a third antihypertensive drug of a different class if the target BP is not reached.[2] Younger children may require higher doses as mg/kg basis than older children.[14]
Many of the options are available for the treatment of high BP, but shortly after the occurrence of an acute CV event, guidelines suggest ACE inhibitor therapy that is titrated upward at short intervals, every 1-2 weeks, until the target BP has been reached [Table 1].[14] For patients who have previously discontinued ACE inhibitor therapy due to intolerance or allergy, an ARB should be substituted.[3] Combined ACE inhibitor/ARB therapy is not recommended, as the combination was associated with an increase in serious adverse effects, but no greater benefit.[15] The combination of a beta-blocker and a thiazide diuretic is less effective than the combination of a CCB and an ACE inhibitor for controlling elevated BP and preventing stroke and CV disease.[16]
Table 1.
Antihypertensive drugs commonly used in hypertensive patients with cardiovascular disease and heart failure
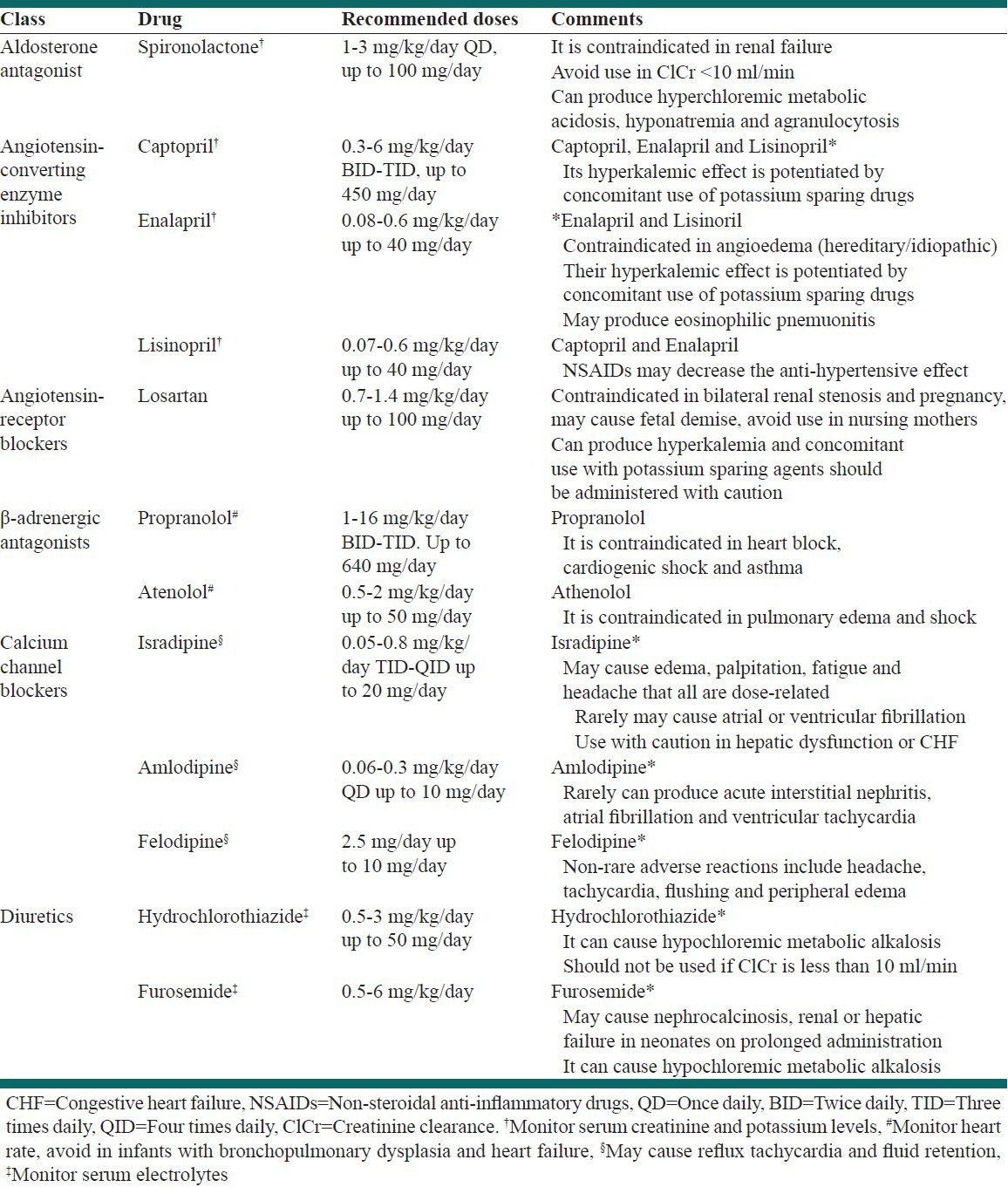
HTN is also a strong predictor of stroke and treating HTN is a well-documented means of primary stroke prevention. There is a strong evidence that antihypertensive therapy is effective for the prevention of recurrent stroke.[17] Another strong predictor of stroke in patients is visit-to-visit variability in systolic BP.[18] This fluctuation in BP differs from “white-coat” HTN and reflects actual changes in BP that escalate with age and are more common among women.
Based on 2010 American Heart Association/American Stroke Association guidelines, in patients with a history of stroke or transient ischemic attack.[19]
Studies suggest benefits from an average BP reduction of approximately 10/5 mm Hg; Joint National Committee seven has defined target BP levels as <120/80 mm Hg
Comprehensive antihypertensive therapy should include salt restriction; weight loss; consumption of a diet rich in fruits, vegetables and low-fat dairy products; regular aerobic physical activity and limited alcohol consumption
Individualize drug therapy according to pharmacologic properties, mechanisms of action and indications based on patient characteristics (e.g., renal impairment, CV disease and diabetes).
TREATMENT OF HYPERTENSIVE PATIENTS WITH HEART FAILURE
Although high BP is a common cause of congestive heart failure (CHF), it also is a predictor of better survival in patients with CHF. This is probably because more severe cardiac dysfunction causes a decline in systemic BP, making low BP a marker for more advanced CHF and the bigger issue in terms of survival. Consequently, HTN remains an important target in patients with CHF since HTN imposes an increased hemodynamic load on the failing ventricle. Several classes of drugs have been shown to prolong survival in patients with CHF, including ACE inhibitors, ARBs, beta-blockers and aldosterone antagonists.[20,21,22]
The stage of CHF is an important consideration for therapy, based on the 2009 American College of Cardiology Foundation/American Heart Association guideline update.[21] For patients with HTN, CV disease or diabetes, staging is as follows:
Stage A: No impaired LV function, hypertrophy
Stage B: Asymptomatic patients with evidence of LV hypertrophy and/or impaired LV function
Stage C: Patients with current or past symptoms of CHF associated with underlying structural heart disease (the bulk of patients with CHF)
Stage D: Patients with truly refractory CHF who might be eligible for mechanical circulatory support.
Recommended therapy
Of all the available therapies for these patients, ACE-inhibitor therapy is the one recommended for all patients with current or prior symptoms of CHF and reduced LV ejection fraction (LVEF). In addition, one of the 3 beta-blockers proven to reduce mortality (bisoprolol, carvedilol and sustained- release metoprolol succinate) is recommended for all stable patients with current or prior symptoms of CHF and reduced LVEF.
Starting therapy with an ACE inhibitor is recommended before beta-blockade is implemented. Begin with low doses (e.g., enalapril 0.08 mg/kg/day or captopril 0.3 mg/kg/dose twice or trice daily) to reduce the likelihood of hypotension and azotemia [Table 1]. If initial therapy is tolerated, increase the dose gradually to maximum doses of 0.6 mg/kg/day of enalapril (up to 40 mg/day), 6 mg/kg/dose 3 times daily of captopril (up to 450 mg/day), unless side-effects occur.[13,14]
Systolic dysfunction
About half of CHF patients have systolic dysfunction or low cardiac output. They develop the classical symptoms of CHF, including edema. Systolic dysfunction is best treated with beta-blockers, ACE inhibitors ARB and spironolactone [Table 1] plus a low-sodium diet.[13,14]
Diastolic dysfunction
These patients have a normal systolic ejection fraction with a stiff or LV hypertrophy (LVH) that can't take in adequate blood, leading to diastolic dysfunction. Echocardiography is usually necessary to make the distinction between those patients with systolic dysfunction and those with a normal systolic ejection fraction.
The major causes of diastolic heart failure are chronic HTN with LVH, hypertrophic cardiomyopathy, aortic stenosis with a normal LVEF and coronary heart disease.
Asymptomatic diastolic dysfunction is more prevalent than symptomatic disease. Symptomatic dysfunction may be associated with typical CHF symptoms, such as reduced exercise capacity and neurohumoral activation, although these are generally less severe than in patients with systolic dysfunction. They are also subject to flash pulmonary edema.
For diastolic dysfunction, drugs that slow heart rate may diminish the stiffness, but otherwise there is no firm set of therapeutic strategies. Regression of LVH is an important therapeutic goal, since it may improve diastolic function. ARB, calcium channel blockers and ACE inhibitors tend to produce significantly more regression than beta-blockers and may be the preferred choices for management of HTN in these patients.
CHF and refractory volume overload
Volume overload is typically addressed with loop diuretics, but a challenging subset of CHF patients exhibits fluid overload despite significant doses of loop diuretics.
In a landmark study, investigators reported that, use of any of several thiazide-type diuretics in combination with a loop diuretic can be more effective than loop diuretic monotherapy in CHF patients with refractory fluid overload [Table 1].[13,18] They showed greater weight loss. The synergistic effects on diuresis appear to be a class effect seen with all thiazide-type diuretics studied.
However, combination therapy comes with a risk of inducing severe hypokalemia, hyponatremia, hypotension and worsening renal function, all warranting close laboratory monitoring.
To avoid the adverse effects of combined diuretic therapy, the study makes the following recommendations:[19]
Combination therapy is only appropriate for patients with gross fluid overload refractory to optimized doses of intravenous (IV) loop diuretics, especially patients with chronic decompensated systolic CHF and impaired renal function
Adequate doses of loop diuretic can be defined as furosemide 160 to 320 mg/d IV in divided doses or by continuous infusion
Carefully selected patients with advanced, refractory, or end-stage (Stage D) systolic CHF may be candidates for outpatient combination diuretic therapy as a means to prevent the recurrent hospitalization for fluid overload, although this approach is not well studied and requires close follow-up.
MANAGEMENT CONSIDERATION IN SPECIAL CONTEXTS AORTIC COARCTATION
Systemic HTN is the main presentation of aortic coarcation that includes 5-10% of all congenital heart defects. The stenosis causing the coractation and HTN is best treated either by transcatheter stent implanatation or surgical correction.[23,24] If severe symptoms are present, labetolol or esmolol is recommended in children with coarctation before definitive surgical or tarnscatheter procedure.[25] However, this disorder, even if primarily treated at an early age with no residual coarctation, can still produce late systemic HTN in approximately 75% of cases 20 to 30 years following the surgical correction.[25,26]
After cardiac transplantation
Majority of patients after cardiac transplantation (96%) develop systemic HTN. ACE inhibitors or diuretics are suggested as probably the first-line treatments in these patients. Beta blockers are allowed to be used in those with refractory HTN despite optimal therapy with aforementioned drugs.[27]
Systemic HTN and aortic dissection
Although a minority of patients with HTN experience aortic dissection, it is still both a risk factor of aortic dissection and a consequent of aortic dissection as a result of renal ischemia or renovascular HTN. Refractory HTN in patients with aortic dissection may be caused by dissection flap. In the context of congenital heart disease, it should be remembered that Marfan syndrome, Loeys-Dietz aneurysm syndrome, bicuspid aortic valve and Turner syndrome all can be associated with aortic aneurysm and dissection.[28]
Co-existence of cardiac and renal abnormalities
Congenital heart diseases are often associated with congenital renal structural anomalies.[29] This coexistence may occur as a recognized pattern such as existence of coarctation and renal anomalies in Turner syndrome.[30] Therefore on dealing with systemic HTN in children with CV disease (CVD), particularly those with congenital heart disease, we should always bear in mind that renal anomalies may coexist with various congenital heart diseases [Figures 1 and 2].
Figure 1.
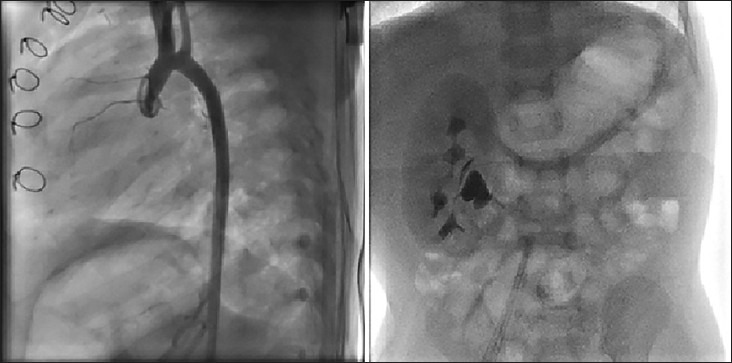
Left panel: Shows the aortogram in lateral view in a 1-year-old male infant after surgical correction for interrupted aortic arch and large ventricular septal defect at the age of 6 months. The abdominal fluoroscopy at the end of the procedure showed lack of any functioning left kidney. Right panel: Renal ultrsonography shows left non-functioning multicystic cystic kidney disease. This patient had normal blood urea nitrogen and creatinine both before and 24 h after the cardiac catheterization and angiography
Figure 2.
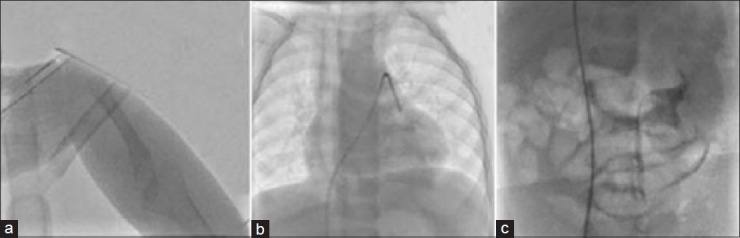
Radiographic imaging of an 18-month-old female infant with Holt-Oram syndrome, very large secundum type atrial septal defect extending to vicinity of superior vena cava. (a) Absent radius of the right hand. (b) Abnormally dilated esophagus on anteroposterior view during cardiac catheterization. (c) Fluoroscopy of abdomen during cardiac catheterization showed no evidence of a functioning right kidney. Abdominal ultrasonography revealed unilateral right-sided renal agenesis
Transcatheter options for treatment of systemic HTN
Until date, there are mainly two transcatheter modalities for treatment of HTN. The first is endovascular stenting for patients with coarcation, middle aortic syndrome, renal artery stenosis and aortic dissection and the second is catheter ablation of renal sympathetic nerves in adults with resistant HTN. However, catheter ablation of renal sympathetic nerves has not yet been reported in the pediatric population.[31]
In summary, treatment of systemic HTN in children is tailored, depending on the pathophysiology mechanism/s, status of systolic or diastolic function of the heart, severity of HTN, presence of end-organ damage and coexisting renal abnormalities.[32]
CONCLUSIONS
Treatment of systemic HTN in children is tailored, depending on the pathophysiology mechanism/s, status of systolic or diastolic function of the heart, severity of HTN, presence of end-organ damage and coexisting renal abnormalities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Falkner B, Daniels SR. Summary of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Hypertension. 2004;44:387–8. doi: 10.1161/01.HYP.0000143545.54637.af. [DOI] [PubMed] [Google Scholar]
- 3.Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, et al. Carotid intima-media thickness in children with CKD: Results from the CKiD study. Clin J Am Soc Nephrol. 2012;7:1930–7. doi: 10.2215/CJN.03130312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urbina EM, Dabelea D, D’Agostino RB, Jr, Shah AS, Dolan LM, Hamman RF, et al. Effect of Type 1 Diabetes on Carotid Structure and Function in Adolescents and Young Adults: The SEARCH CVD study. Diabetes Care. 2013;36:2597–9. doi: 10.2337/dc12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels SR. Obesity, vascular changes, and elevated blood pressure. J Am Coll Cardiol. 2012;60:2651–2. doi: 10.1016/j.jacc.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Assadi F. Relation of left ventricular hypertrophy to microalbuminuria and C-reactive protein in children and adolescents with essential hypertension. Pediatr Cardiol. 2008;29:580–4. doi: 10.1007/s00246-007-9153-4. [DOI] [PubMed] [Google Scholar]
- 7.Sprecher DL, Schaefer EJ, Kent KM, Gregg RE, Zech LA, Hoeg JM, et al. Cardiovascular features of homozygous familial hypercholesterolemia: Analysis of 16 patients. Am J Cardiol. 1984;54:20–30. doi: 10.1016/0002-9149(84)90298-4. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Takahashi O, et al. Coronary arterial lesions of Kawasaki disease: Cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7:3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 9.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: The original heart failure syndrome. Eur Heart J. 2003;24:970–6. doi: 10.1016/s0195-668x(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 10.Matteucci MC, Wühl E, Picca S, Mastrostefano A, Rinelli G, Romano C, et al. Left ventricular geometry in children with mild to moderate chronic renal insufficiency. J Am Soc Nephrol. 2006;17:218–26. doi: 10.1681/ASN.2005030276. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JT. Hypertension in the young: Epidemiology, sequelae and therapy. Nephrol Dial Transplant. 2009;24:370–5. doi: 10.1093/ndt/gfn597. [DOI] [PubMed] [Google Scholar]
- 12.Daniels SR, Pratt CA, Hayman LL. Reduction of risk for cardiovascular disease in children and adolescents. Circulation. 2011;124:1673–86. doi: 10.1161/CIRCULATIONAHA.110.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thune JJ, Signorovitch J, Kober L, Velazquez EJ, McMurray JJ, Califf RM, et al. Effect of antecedent hypertension and follow-up blood pressure on outcomes after high-risk myocardial infarction. Hypertension. 2008;51:48–54. doi: 10.1161/HYPERTENSIONAHA.107.093682. [DOI] [PubMed] [Google Scholar]
- 14.Lande MB, Flynn JT. Treatment of hypertension in children and adolescents. Pediatr Nephrol. 2009;24:1939–49. doi: 10.1007/s00467-007-0573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 17.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 19.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 20.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–34. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Assadi F. The growing epidemic of hypertension among children and adolescents: A challenging road ahead. Pediatr Cardiol. 2012;33:1013–20. doi: 10.1007/s00246-012-0333-5. [DOI] [PubMed] [Google Scholar]
- 22.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 23.Sadiq M, Rehman AU, Qureshi AU, Qureshi SA. Covered stents in the management of native coarctation of the Aorta-Intermediate and long-term follow-up. Catheter Cardiovasc Interv. 2013;82:511–8. doi: 10.1002/ccd.24945. [DOI] [PubMed] [Google Scholar]
- 24.Forbes T, Matisoff D, Dysart J, Aggarwal S. Treatment of coexistent coarctation and aneurysm of the aorta with covered stent in a pediatric patient. Pediatr Cardiol. 2003;24:289–91. doi: 10.1007/s00246-002-0262-9. [DOI] [PubMed] [Google Scholar]
- 25.Buys R, Van De Bruaene A, Müller J, Hager A, Khambadkone S, Giardini A, et al. Usefulness of cardiopulmonary exercise testing to predict the development of arterial hypertension in adult patients with repaired isolated coarctation of the aorta. Int J Cardiol. 2013;168:2037–41. doi: 10.1016/j.ijcard.2013.01.171. [DOI] [PubMed] [Google Scholar]
- 26.Canniffe C, Ou P, Walsh K, Bonnet D, Celermajer D. Hypertension after repair of aortic coarctation-A systematic review. Int J Cardiol. 2013;167:2456–61. doi: 10.1016/j.ijcard.2012.09.084. [DOI] [PubMed] [Google Scholar]
- 27.Roche SL, O’Sullivan JJ, Kantor PF. Hypertension after pediatric cardiac transplantation: Detection, etiology, implications and management. Pediatr Transplant. 2010;14:159–68. doi: 10.1111/j.1399-3046.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 28.Braverman AC. Acute aortic dissection: Clinician update. Circulation. 2010;122:184–8. doi: 10.1161/CIRCULATIONAHA.110.958975. [DOI] [PubMed] [Google Scholar]
- 29.Cruz DN, Bagshaw SM. Heart-kidney interaction: Epidemiology of cardiorenal syndromes. Int J Nephrol 2010. 2011 doi: 10.4061/2011/351291. 351291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho AB, Guerra G, Júnior, Baptista MT, de Faria AP, Marini SH, Guerra AT. Cardiovascular and renal anomalies in Turner syndrome. Rev Assoc Med Bras. 2010;56:655–9. doi: 10.1590/s0104-42302010000600012. [DOI] [PubMed] [Google Scholar]
- 31.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–81. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 32.Severin PN, Awad S, Shields B, Hoffman J, Bonney W, Cortez E, et al. The pediatric cardiology pharmacopeia: 2013 update. Pediatr Cardiol. 2013;34:1–29. doi: 10.1007/s00246-012-0553-8. [DOI] [PubMed] [Google Scholar]


