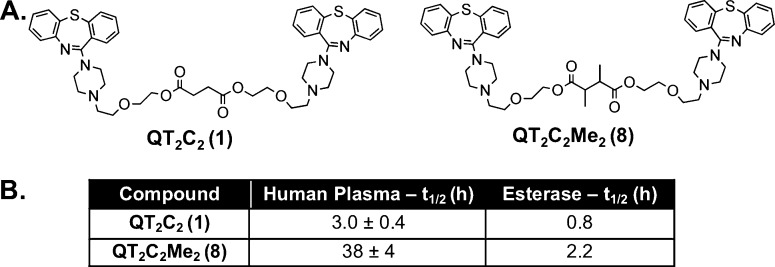
Figure 1.
Structures of QT2C2 (1) and QT2C2Me2 (8) and stability analysis. (A) The hindered dimer prodrug, QT2C2Me2 (8), was synthesized as in Scheme 1 using a dicarboxylic acid linker (2,3-dimethylsuccinic acid). The prodrugs were purified to homogeneity by reverse phase HPLC, purity confirmed by analytical HPLC, and structures confirmed by MALDI-TOF mass spectrometry. (B) QT2C2 (1) and QT2C2Me2 (8) (40 μM each) were incubated in the presence of human plasma (55% in 10 mM phosphate buffer, pH 7.4) or 10 units of pig liver esterase at 37 °C. Reversion of the prodrugs to monomer was quantified via analytical HPLC and normalized to an internal standard. t1/2 values for stability in human plasma represent duplicate experiments ± SD.