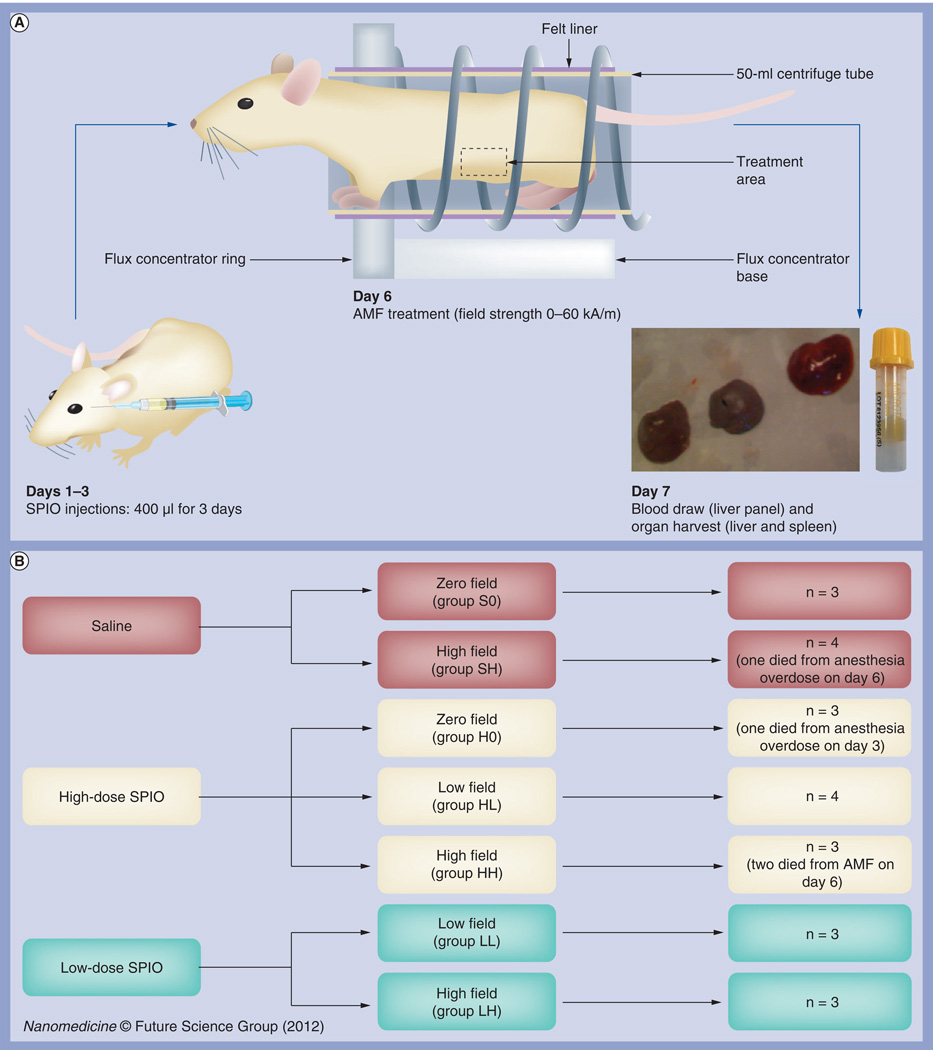
Figure 1. Experimental design.
(A) Flow chart of experimental design. Twenty three mice were subjected to daily systemic magnetic dextran–iron oxide injections (at zero, low or high doses) on days 1–3 and exposed to AMF on day 6 (at zero-, low- or high-field strengths). Blood was collected and organs harvested on day 7 for data analysis. (B) Mouse group designations. Mice were randomly assigned to seven groups of three to four mice each, including three control groups (S0, SH and H0) and four treatment groups (LL, LH, HL and HH). The first letter of each group designation indicates the dose of nanoparticles injected: saline (S); low-dose (L); or high-dose (H) SPIO. The second letter of each group designation indicates the field strength for AMF exposure, including zero (0), low (L) and high (H) field. The doses used were 1.65 mg total iron content for low-dose SPIO and 7.2 mg total iron content for high dose, or equivalent to five- and 20-times the maximum tolerated dose for humans. Field strengths used were 24 kA/m for low field and 60 kA/m for high field.
AMF: Alternating magnetic field; SPIO: Superparamagnetic iron oxide.