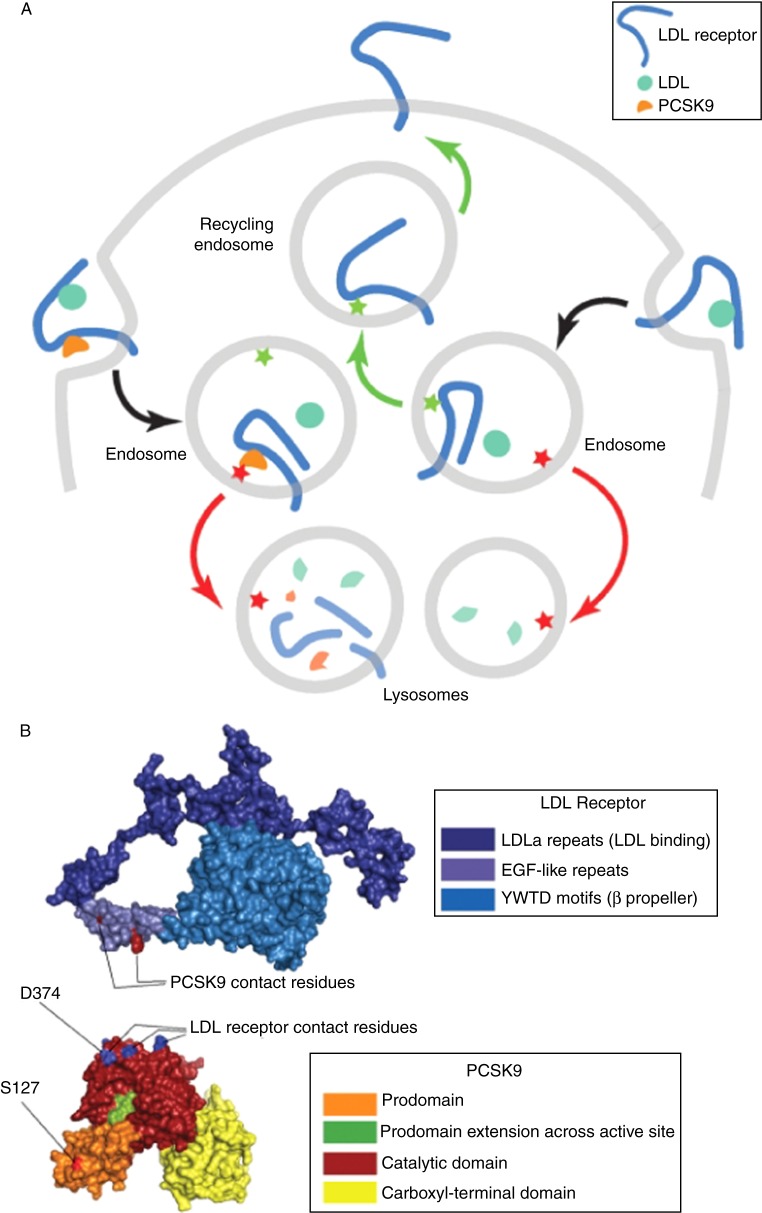
Figure 5.
A: Model of PCSK9-mediated sorting of LDL receptors to lysosomes. The EGFa domain of the LDL receptor is required for proper sorting of the LDL receptor back to the cell surface. The EGFa domain may contain a sorting signal that interacts with an endosomal protein (green star), directing the LDL receptor back to the cell surface on recycling endosomes (green arrows). Binding of PCSK9 might interfere with that signal, preventing the LDL receptor from returning to the cell surface. Alternatively, PCSK9 could contain a distinct sorting signal (red star) that results in the sorting of the PCSK9-LDL receptor complex (red arrows) to lysosomes. The gain-of-function mutation involving S127 of PCSK9 may enhance the sorting of the PCSK9-LDL receptor complex to lysosomes. B: The structure of the LDL receptor and PCSK9 at endosomal pH. The LDL receptor is folded back upon itself at low pH; however, the face of the EGFa domain that binds PCSK9 is exposed. The LDL receptor-binding site on PCSK9 is at the apex of a roughly triangular structure formed by the tripartite domain structure of PCSK9. The D374 residue that is altered in gain-of-function PCSK mutants is located within the apical LDL receptor-binding site, whereas the S127 residue is quite distant from the binding interface. S127 mutations do not affect binding of PCSK9 to the LDL receptor. Gain-of-function mutations affecting residue 127 may reduce LDL receptors by enhancing the sorting of LDL receptors to lysosomes, rather than by affecting the strength of PCSK9-LDL receptor interactions. (figure and accompanying legend from Peterson A, Fong L and Young S. PCSK9 function and Physiology. J Lipid Res. 2008 June; 49(6): 1152–1156).