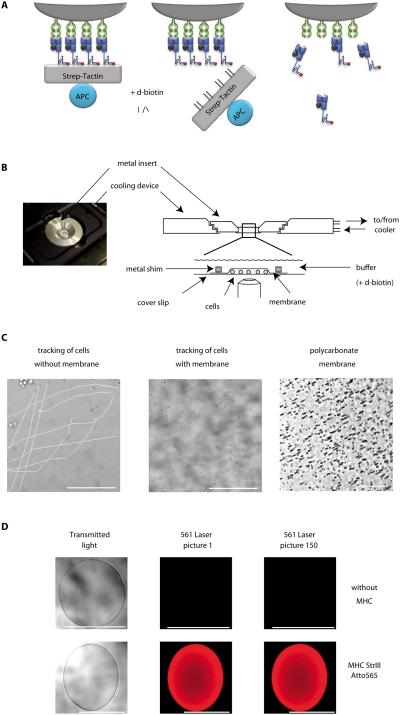
Figure 1. Basic principle and experimental realization of the koff-rate assay.
(A) Specific CD8+ T cells are stably labeled with dichromatic Streptamers (left). Addition of D-biotin displaces Strep-Tactin, leaving monomeric pMHC molecules bound to surface expressed TCRs (middle). Subsequent dissociation of monomeric MHC molecules is observed as a decay of the Atto565 fluorescence by real-time microscopy. (B) A cooling device is mounted on the microscope (left). Purified T cells are pipetted into a 4°C cooled reservoir build of a metal insert sealed with a cover slip, arrested by a polycarbonate membrane and a metal shim and covered with cold buffer (right). (C) Reduced movement of cells covered with polycarbonate membrane (middle) in comparison to cells not arrested (left) over a time series of 200 pictures after addition of D-biotin. 5μm pores in the membrane allow quick diffusion of buffer (right, dark spots). (D) Validation of Atto565 fluorescence on agarose Strep-Tactin beads in the koff-rate setup. Transmitted light and Atto565 fluorescence of unloaded beads (upper row) or beads loaded with Atto565 labeled MHC molecules (lower row) of two representative experiments. Scale bars indicate 100μm.