Figure 2.
Jmjd4 Catalyzes 2OG- and Fe(II)-Dependent C4 Lysyl Hydroxylation of eRF1
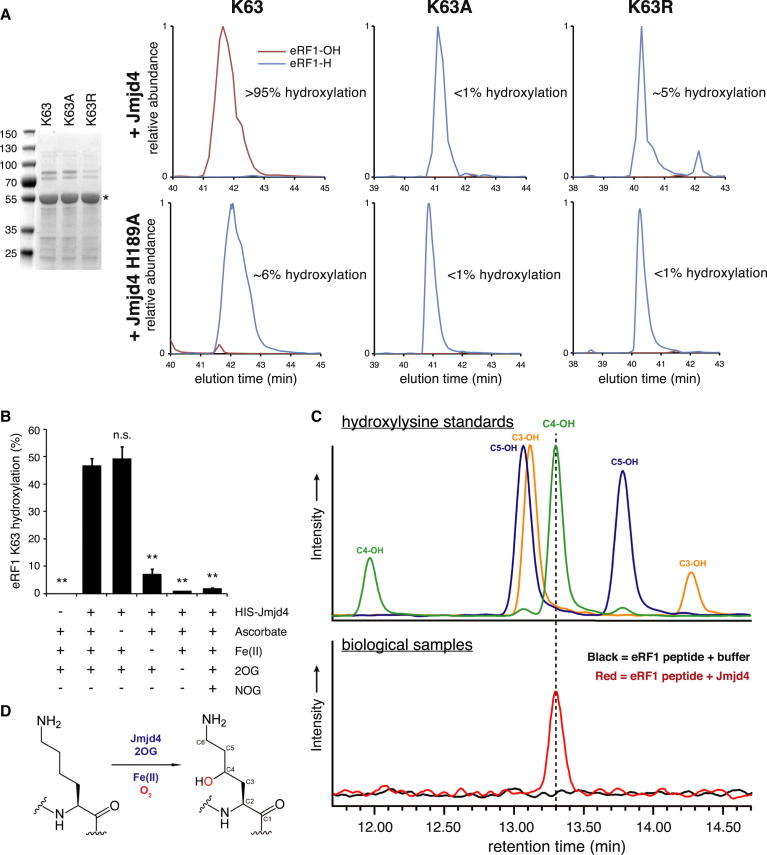
(A) K63 mutation prevents Jmjd4-dependent hydroxylation of eRF1. Left: Coomassie gel of partially purified recombinant eRF1 and mutants. Right: LC-MS extracted ion chromatograms (EICs) show wild-type and mutant eRF1 reacted with either wild-type (top row) or mutant H189A (bottom row) Jmjd4. ([M+H]2+; K63-H: m/z 698.842; K63-OH: m/z 706.840; K63R-H: m/z 712.846; K63R-OH: m/z 720.843; K63A-H: m/z 791.880; K63A-OH: m/z 799.878).
(B) Jmjd4 is a 2OG/Fe(II)-dependent oxygenase. In vitro assays were performed in the presence or absence of the indicated cofactors and inhibitors. 2OG oxygenases are competitively inhibited by NOG, a nonhydrolysable form of 2OG. Data represent mean ± SEM. Statistical significance was evaluated by ANOVA followed by Dunett’s post hoc test, comparing all treatments to the reaction complemented with all cofactors (n.s., not significant; ∗∗p < 0.01).
(C) Jmjd4 is a C4 lysyl hydroxylase. Bottom: a cyclic thioether-linked dimer of a 15-mer peptide containing eRF1 residues 57–70 was untreated (buffer, black) or Jmjd4 hydroxylated (red) prior to hydrolysis and LC-MS. Top: chromatography peaks observed in biological samples were identified with C3, C4, and/or C5 hydroxylysine standards. Note that two peaks are observed because each standard is a mixture of stereoisomers. See Figure S2 for NMR of standards and further validation of the C4 assignment.
(D) Schematic of C4 lysyl hydroxylation catalyzed by Jmjd4. See also Figure S2.