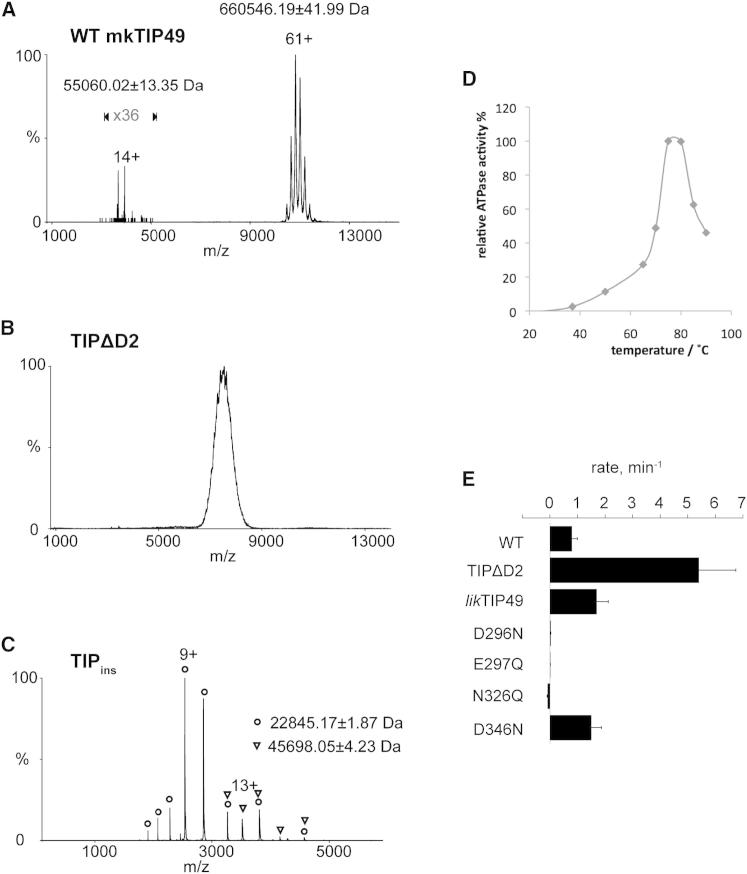
Figure 4.
mkTIP49 Oligomeric State and ATP Turnover
(A–C) Mass spectrometry analysis of wild-type mkTIP49, of TIPΔD2, and of the isolated D2 domain (TIPins, residues 124–290) is shown in (A), (B), and (C), respectively.
(D) The optimal temperature range for ATP hydrolysis by the wild-type mkTIP49 protein.
(E) Comparison of ATP turnover between the wild-type, TIPΔD2, likTIP49, and catalytic-site mutant proteins. The ATP turnover of mkTIP was determined at temperatures of 37°C, 50°C, 65°C, 70°C, 75°C, 80°C, 85°C, and 90°C as described in the Supplemental Experimental Procedures. Measurements were repeated 2–4 times, and the plotted values show a representative experiment within the full temperature range. See also Figure S1 for the location of mutations within the amino-acid sequence of mkTIP49.